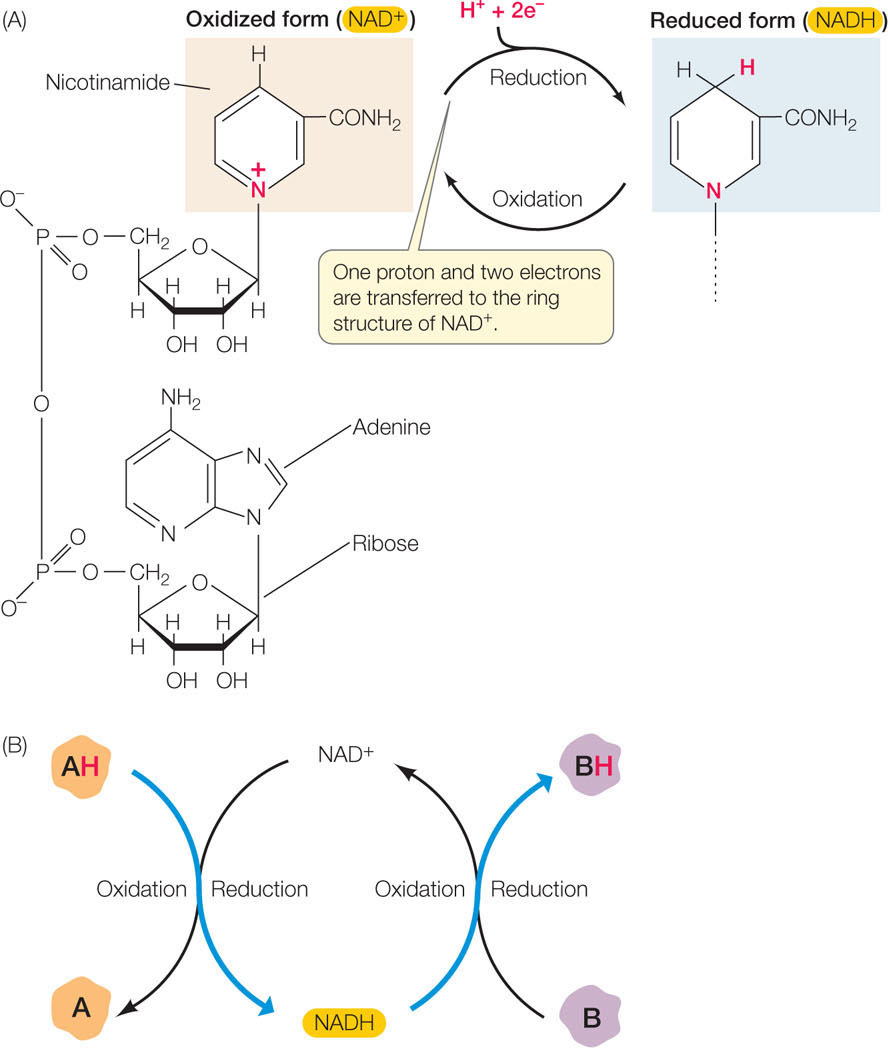
Figure 6.4: NAD+/NADH Is an Electron Carrier in Redox Reactions (A) NAD+ is an important electron acceptor in redox reactions, and its reduced form, NADH, is an important energy intermediary in cells. The unshaded portion of the molecule (left) remains unchanged by the redox reaction. (B) Coupling of redox reactions using NAD+/NADH.