Concept 2.2: Atoms Interact and Form Molecules
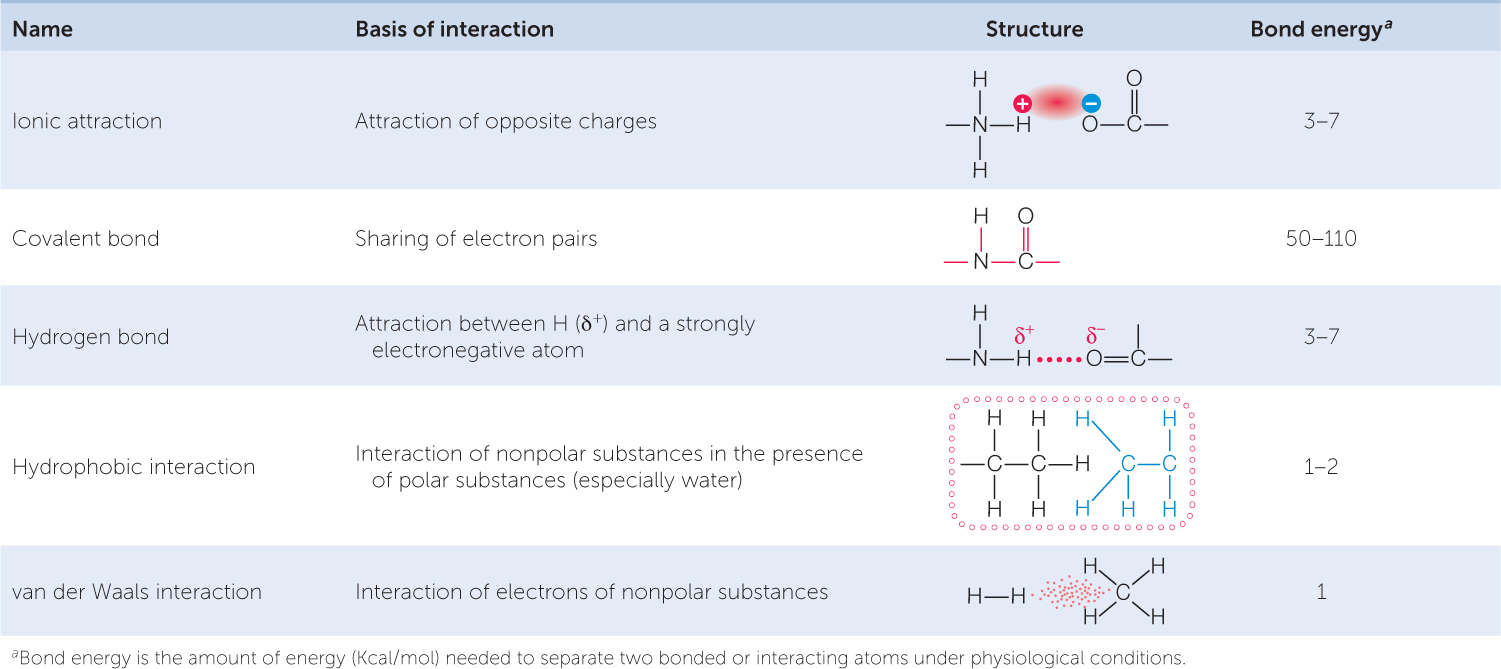
A chemical bond is an attractive force that links two atoms together in a molecule. There are several kinds of chemical bonds (TABLE 2.1). In this section we will begin with covalent bonds, the strong bonds that result from the sharing of electrons. Next we will consider weaker interactions, including hydrogen bonds, which are enormously important to biology. We will then examine ionic attractions, which form when an atom gains or loses electrons to achieve stability. Finally, we will see how atoms are bonded to make functional groups—groups of atoms that give important properties to biological molecules.

Go to ANIMATED TUTORIAL 2.1 Chemical Bond Formation
PoL2e.com/at2.1
Covalent bonds consist of shared pairs of electrons
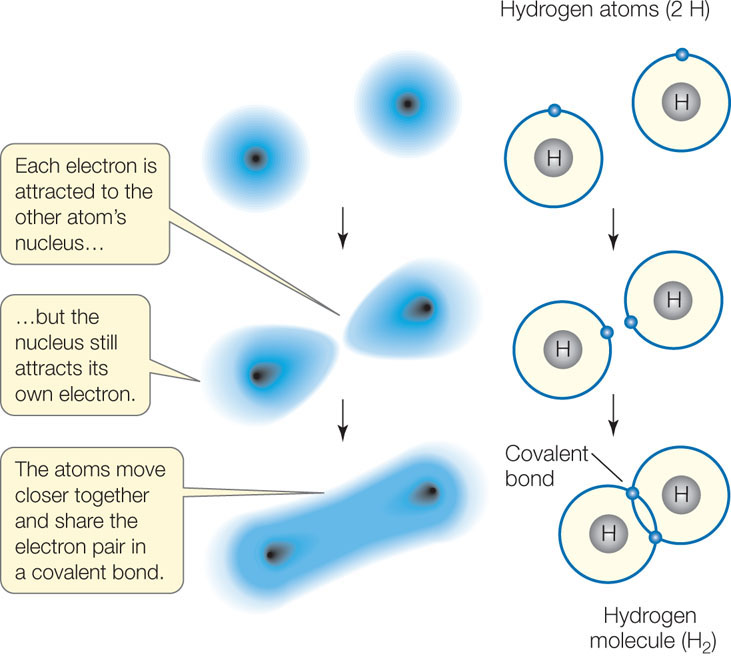
A covalent bond forms when two atoms attain stable electron numbers in their outermost shells by sharing one or more pairs of electrons. In this case, each atom contributes one member of each electron pair. Consider two hydrogen atoms coming close together, each with just one electron in its single shell (FIGURE 2.2). When the electrons pair up, a stable association is formed, and this links the two hydrogen atoms in a covalent bond, forming the molecule H2.
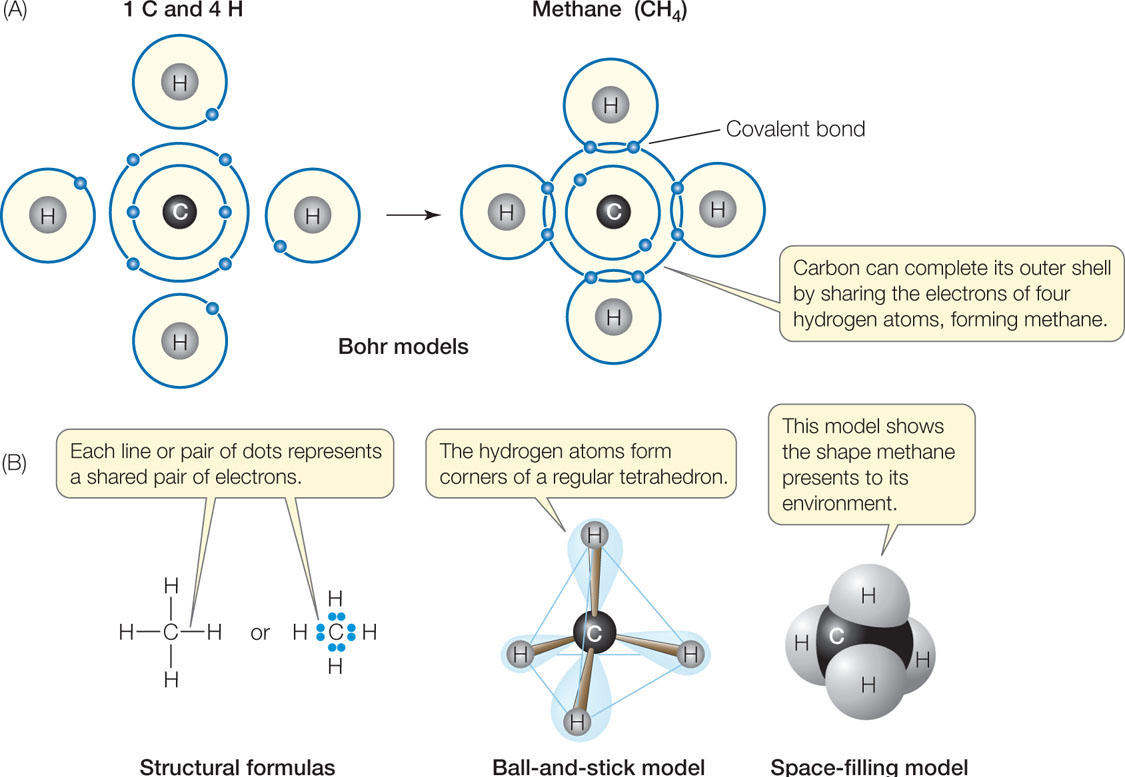
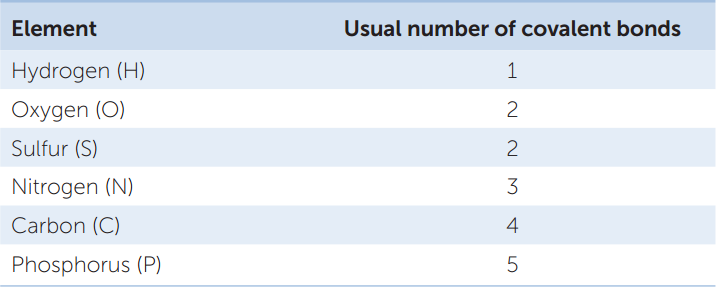
Let’s see how covalent bonds are formed in the somewhat more complicated methane molecule (CH4). The carbon atom has six electrons: two electrons fill its inner shell, and four electrons are in its outer shell. Because of the octet rule, carbon is most stable when it shares electrons with four other atoms—it can form four covalent bonds (FIGURE 2.3A). Methane forms when an atom of carbon reacts with four hydrogen atoms. As a result of electron sharing, the outer shell of the carbon atom is now filled with eight electrons—a stable configuration. The single shell of each hydrogen atom is also filled. Four covalent bonds—four shared electron pairs—hold methane together. FIGURE 2.3B shows several different ways to represent the molecular structure of methane. TABLE 2.2 shows the covalent bonding capacities of some biologically important elements.
21
The properties of molecules are influenced by the characteristics of their covalent bonds. Four important aspects of covalent bonds are orientation, strength and stability, multiple covalent bonds, and the degree of sharing of electrons.
Orientation
For a given pair of elements, such as carbon bonded to hydrogen, the length of the covalent bond is always the same. And for a given atom within a molecule, the angle of each covalent bond with respect to the others is generally the same. This is true regardless of the type of larger molecule that contains the atom. For example, the four covalent bonds formed by the carbon atom in methane are always distributed in space so that the bonded hydrogens point to the corners of a regular tetrahedron, with the carbon in the center (see Figure 2.3B). Even when carbon is bonded to four atoms other than hydrogen, this three-dimensional orientation is more or less maintained. As you will see, the orientations of covalent bonds in space give molecules their three-dimensional geometry, and the shapes of molecules contribute to their biological functions.
Strength and Stability
Covalent bonds are very strong (see Table 2.1), meaning it takes a lot of energy to break them. At the temperatures at which life exists, the covalent bonds of biological molecules are quite stable, as are their three-dimensional structures. However, this stability does not rule out change, as we will discover.
Multiple Covalent Bonds
As shown in Figure 2.3B, covalent bonds can be represented by lines between the chemical symbols for the linked atoms:
- A single bond involves the sharing of a single pair of electrons (for example, H—H or C—H).
- A double bond involves the sharing of four electrons (two pairs; C
 C).
C). - Triple bonds—six shared electrons—are rare, but there is one in nitrogen gas (N
 N), which is the major component of the air we breathe.
N), which is the major component of the air we breathe.
Unequal Sharing of Electrons
If two atoms of the same element are covalently bonded, there is an equal sharing of the pair(s) of electrons in their outermost shells. However, when the two atoms are different elements, the sharing is not necessarily equal. One nucleus may exert a greater attractive force on the electron pair than the other nucleus, so that the pair tends to be closer to that atom.
The attractive force that an atomic nucleus exerts on electrons in a covalent bond is called its electronegativity. The electronegativity of a nucleus depends on how many positive charges it has (nuclei with more protons are more positive and thus more attractive to electrons) and on the distance between the electrons in the bond and the nucleus (the closer the electrons, the greater the electronegative pull). TABLE 2.3 shows the electronegativities of some elements important in biological systems. (Electronegativity is calculated to produce a dimensionless quantity, meaning that it has no unit of measurement such as for length, time, mass, etc.)
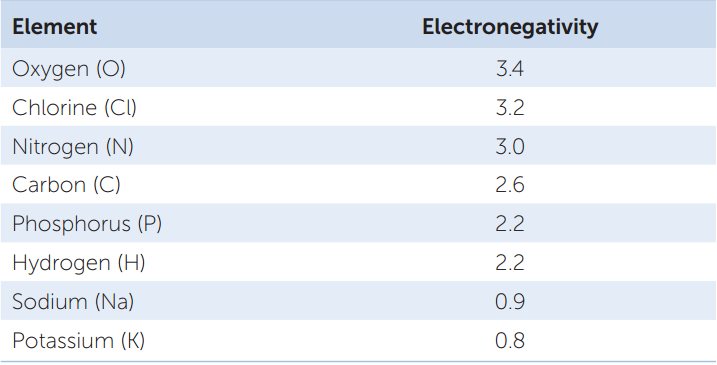
If two atoms are 0.5 or less apart in electronegativity, they will share electrons equally in what is called a nonpolar covalent bond. Two oxygen atoms, for example, each with an electronegativity of 3.4, will share electrons equally. So will two hydrogen atoms (each with an electronegativity of 2.2). But when hydrogen bonds with oxygen to form water, the electrons involved are unequally shared: they tend to be nearer to the oxygen nucleus because it is more electronegative than hydrogen. When electrons are drawn to one nucleus more than to the other, the result is a polar covalent bond:
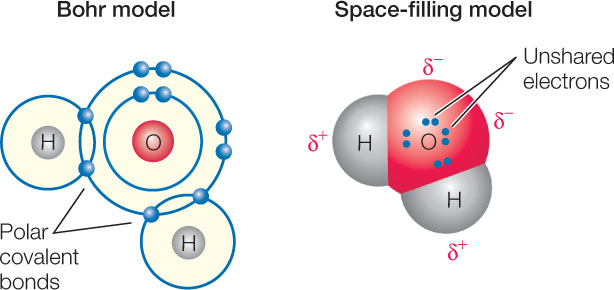
Because of this unequal sharing of electrons, the oxygen end of the bond has a slightly negative charge (symbolized by δ− and spoken of as “delta negative,” meaning a partial unit of charge), and the hydrogen end has a slightly positive charge (δ+). The bond is polar because these opposite charges are separated at the two ends, or poles, of the bond. The partial charges that result from polar covalent bonds produce polar molecules or polar regions of large molecules. Polar bonds within molecules greatly influence the interactions they have with other polar molecules. The polarity of the water molecule has significant effects on its physical properties and chemical reactivity, as we will see in later chapters.
23
Hydrogen bonds may form within or between molecules with polar covalent bonds
In liquid water, the negatively charged oxygen (δ−) atom of one water molecule is attracted to the positively charged hydrogen (δ+) atoms of other water molecules (FIGURE 2.4A). The bond resulting from this attraction is called a hydrogen bond (see Table 2.1). These bonds are not restricted to water molecules. A hydrogen bond may also form between a strongly electronegative atom and a hydrogen atom that is covalently bonded to another electronegative atom (oxygen or nitrogen), as shown in FIGURE 2.4B.
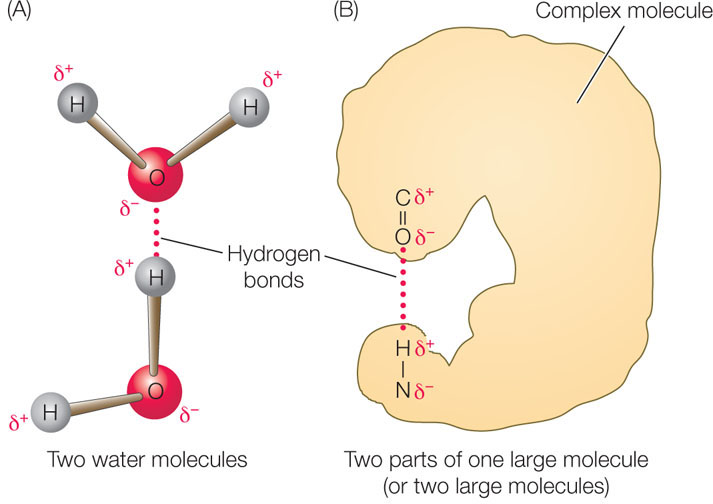
A hydrogen bond is much weaker than a covalent bond (see Table 2.1). Although individual hydrogen bonds are weak, many of them can form within one molecule or between two molecules. In these cases, the hydrogen bonds together have considerable strength and can greatly influence the structure and properties of the substances. Hydrogen bonds play important roles in determining and maintaining the three-dimensional shapes of giant molecules such as DNA and proteins (see Chapter 3).
Hydrogen bonding also contributes to several properties of water that have great significance for life. As we will discuss later in this Concept, water plays a vital role in living systems as a solvent: a liquid in which other molecules dissolve. Other important properties of water are its heat capacity, cohesion, adhesion, and surface tension.
Heat Capacity
At any given time in liquid water, a water molecule forms an average of 3.4 hydrogen bonds (dotted red lines below) with other water molecules:
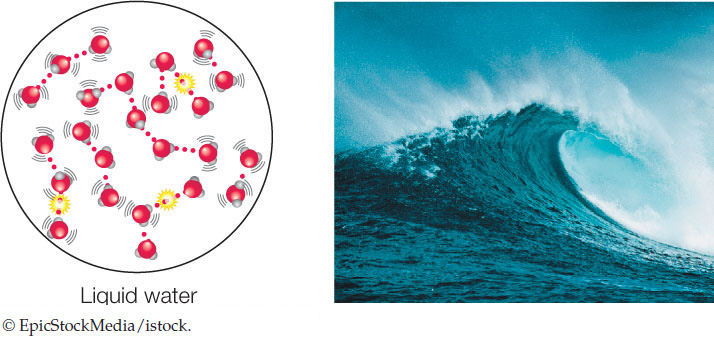
These multiple hydrogen bonds contribute to the high heat capacity of water. Raising the temperature of liquid water takes a lot of heat, because much of the heat energy is used to break the hydrogen bonds that hold the liquid together (indicated by the yellow energy bursts in the liquid water diagram in the previous column). Think of what happens when you apply heat to a pan of water on the stove: it takes a while for the water to begin boiling. The same happens with a living organism—the large amount of water in living tissues shields the organism from fluctuations in environmental temperature.
Hydrogen bonding also gives water a high heat of vaporization. This means that a lot of heat is required to change water from its liquid to its gaseous state, in the process of evaporation. Once again, much of the heat energy is used to break the many hydrogen bonds between the water molecules. This heat must be absorbed from the environment in contact with the water. Evaporation thus has a cooling effect on the environment—whether a leaf, a forest, or an entire land mass. This effect explains why sweating cools the human body: as sweat evaporates from the skin, it absorbs some of the adjacent body heat.
LINK
Evaporation is important in the physiology of both plants and animals; see Concepts 25.3 and 29.4
Cohesion, Adhesion, and Surface Tension
The numerous hydrogen bonds that give water a high heat capacity and high heat of vaporization also explain the cohesive strength of liquid water. This cohesive strength, or cohesion, is defined as the capacity of water molecules to resist coming apart from one another when placed under tension. Hydrogen bonding between liquid water molecules and the solid surfaces surrounding them also allows for adhesion between the water and the solid surfaces. Together, cohesion and adhesion permit narrow columns of liquid water to move from the roots to the leaves of tall trees (see Concept 25.3). When water evaporates from the leaves, the entire column moves upward in response to the pull of the molecules at the top.
24

The surface of liquid water exposed to air is difficult to puncture because the water molecules at the surface are hydrogen-bonded to other molecules below them. This surface tension permits a container to be filled with water above its rim without overflowing, and it permits spiders to walk on the surface of a pond.
Polar and nonpolar substances: Each interacts best with its own kind
Just as water molecules can interact with one another through hydrogen bonds, any polar molecule can interact with any other polar molecule through the weak (δ+ to δ−) attractions of hydrogen bonds. Polar molecules interact with water in this way and are called hydrophilic (“water-loving”). In aqueous (watery) solutions, these molecules become separated and surrounded by water molecules (FIGURE 2.5A). Thus, water functions as a solvent for polar molecules.
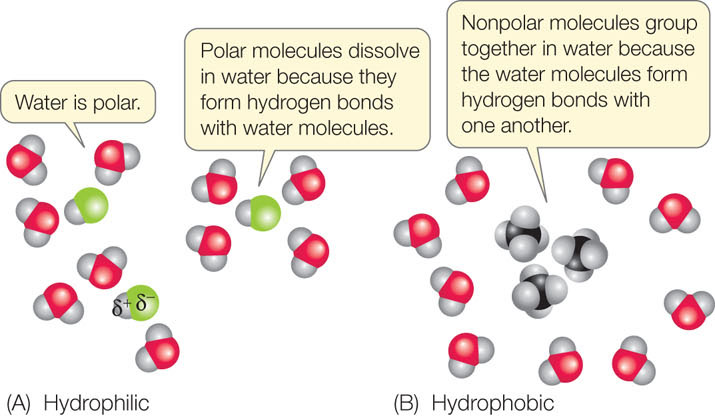
Because they do not have partial charges, nonpolar molecules do not interact with water in this way. Instead, these molecules tend to aggregate with one another. This allows more hydrogen bonds to form between water molecules. Therefore, nonpolar molecules are known as hydrophobic (“water-hating”), and the interactions between them are called hydrophobic interactions (FIGURE 2.5B). Hydrophobic substances do not really “hate” water—they can form weak interactions with it, since the electronegativities of the atoms in many nonpolar bonds (e.g., C—H bonds) are not exactly the same. But these interactions are far weaker than the hydrogen bonds between the water molecules, so the nonpolar substances tend to aggregate.
APPLY THE CONCEPT: Atoms interact and form molecules
The concepts of chemical bonding and electronegativity (see Table 2.2) allow us to predict whether a molecule will be polar or nonpolar, and how it will interact with water. Typically, a difference in electronegativity greater than 0.5 will result in polarity. For each of the bonds below, indicate:
- Whether the bond is polar or nonpolar
- If polar, which is the δ+ end
- How a molecule with the bond will interact with water (hydrophilic or hydrophobic)
N—H
O—H
C—H
C—C
C O
O
H—H
C—N
O—P
Ionic attractions form between anions and cations
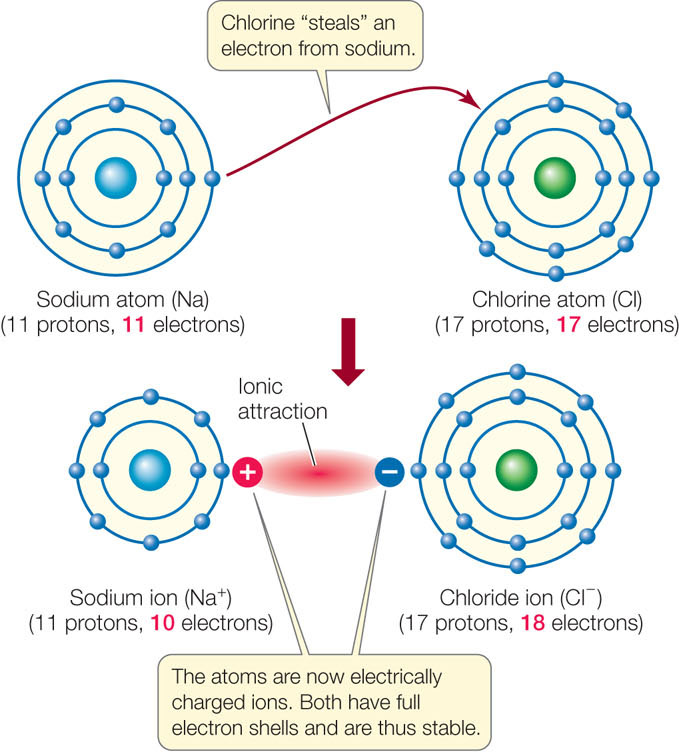
When one interacting atom is much more electronegative than the other (see Table 2.3), a complete transfer of one or more electrons may occur. Consider sodium (11 protons) and chlorine (17 protons). A sodium atom has only one electron in its outermost shell; this condition is unstable. A chlorine atom has seven electrons in its outermost shell—another unstable condition. The most straightforward way for both atoms to achieve stability is to transfer an electron from sodium’s outermost shell to that of chlorine (FIGURE 2.6). This reaction makes the two atoms more stable because they both have eight electrons in their outer shells. The result is two ions.
An ion is an electrically charged particle that forms when an atom gains or loses one or more electrons:
- The sodium ion (Na+) in our example has a charge of +1 because it has one less electron than it has protons. The outermost electron shell of the sodium ion is stable because it has eight electrons. Positively charged ions are called cations.
- The chloride ion (Cl−) has a charge of −1 because it has one more electron than it has protons. This additional electron gives Cl− a stable outermost shell with eight electrons. Negatively charged ions are called anions.
Ionic attractions form as a result of the electrical attraction between ions bearing opposite charges. Ionic attractions result in stable crystalline structures that are often referred to as ionic compounds or salts. An example is sodium chloride (NaCl; table salt), where cations and anions are held together by ionic attractions. Ionic attractions in salt crystals may be stronger, but attractions between ions in solution, as occur in living systems, are typically weak (see Table 2.1).
25
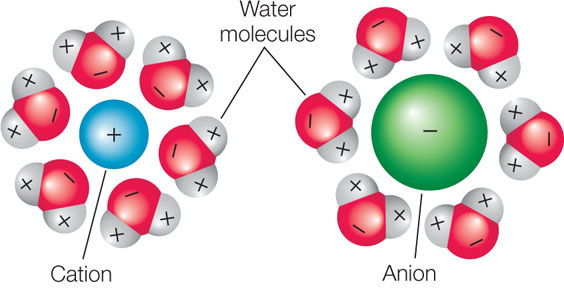
Given that living organisms consist of about 70 percent water, most biological processes occur in the presence of water. Because ionic attractions are weak, salts dissolve in water; the ions separate from one another and become surrounded by water molecules. The water molecules are oriented with their negative poles nearest to the cations and their positive poles nearest to the anions:
Functional groups confer specific properties to biological molecules
Certain small groups of atoms, called functional groups, are consistently found together in very different biological molecules. You will encounter several functional groups repeatedly in your study of biology (FIGURE 2.7). Each functional group has specific chemical properties (for example, polarity), and when attached to a larger molecule, it gives those properties to the larger molecule. The consistent chemical behavior of functional groups helps us understand the properties of the molecules that contain them.
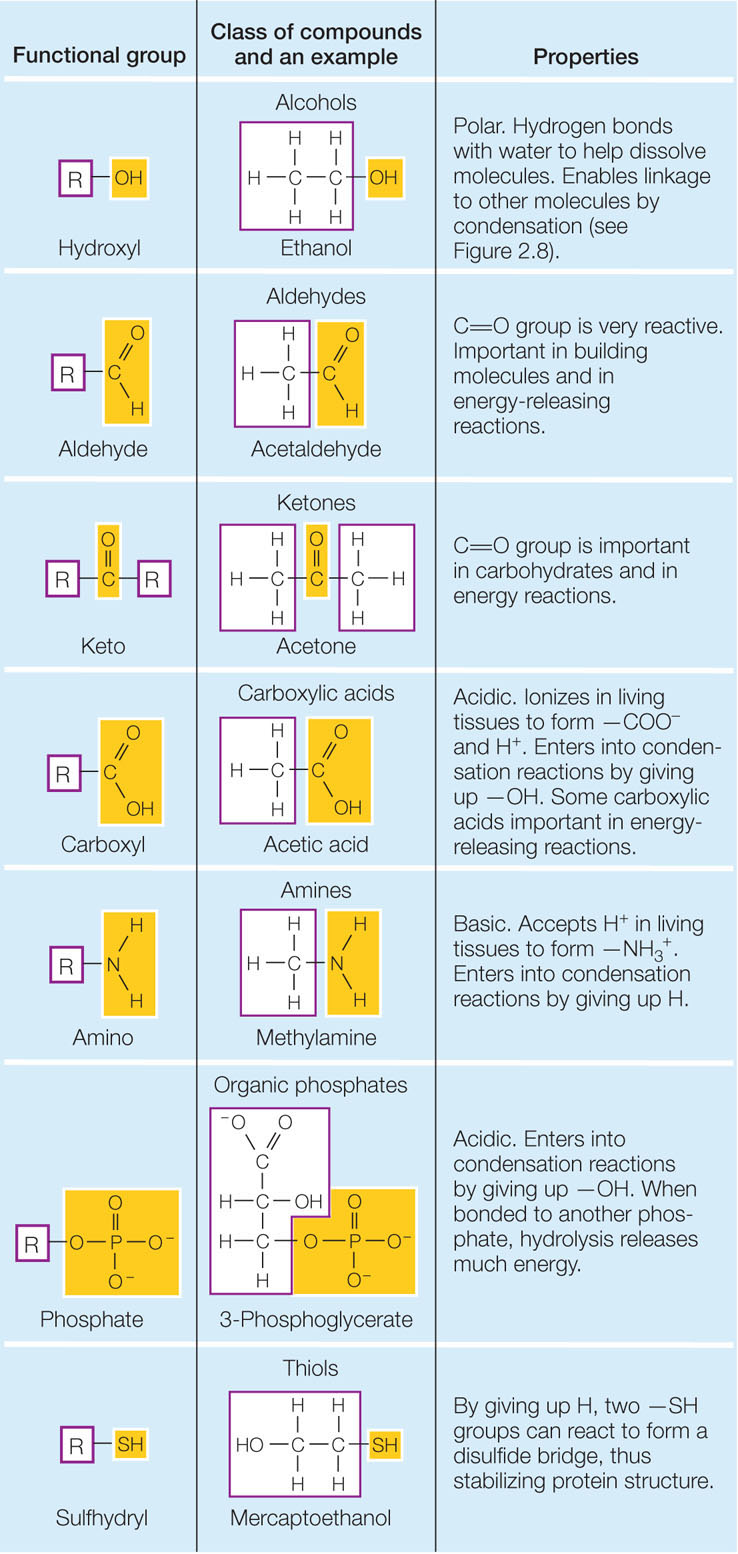
Biological molecules often contain many different functional groups. A single large protein may contain hydrophobic, polar, and charged functional groups. Each group gives a different specific property to its local site on the protein, and it may interact with another functional group on the same protein or with another molecule. The functional groups thus determine molecular shape and reactivity.
26
Go to ACTIVITY 2.1 Functional Groups
PoL2e.com/ac2.1
Macromolecules are formed by the polymerization of smaller molecules
Large molecules, called macromolecules, are formed by covalent linkages between smaller molecules. Four kinds of macromolecules are characteristic of living things: proteins, carbohydrates, nucleic acids, and lipids.
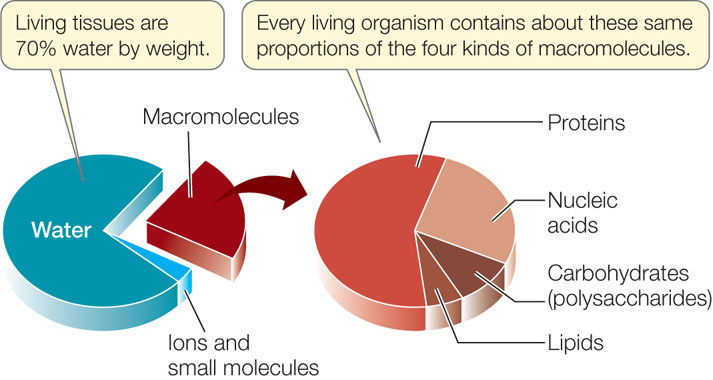
With the exception of small carbohydrates and lipids, these biological molecules are polymers (poly, “many”; mer, “unit”) constructed by the covalent bonding of smaller molecules called monomers.
- Proteins are formed from different combinations of 20 amino acids, all of which share chemical similarities.
- Carbohydrates can be giant molecules, and are formed by linking together chemically similar sugar monomers (monosaccharides) to form polysaccharides.
- Nucleic acids are formed from four kinds of nucleotide monomers linked together in long chains.
- Lipids also form large structures from a limited set of smaller molecules, but in this case noncovalent forces maintain the interactions between the lipid monomers.
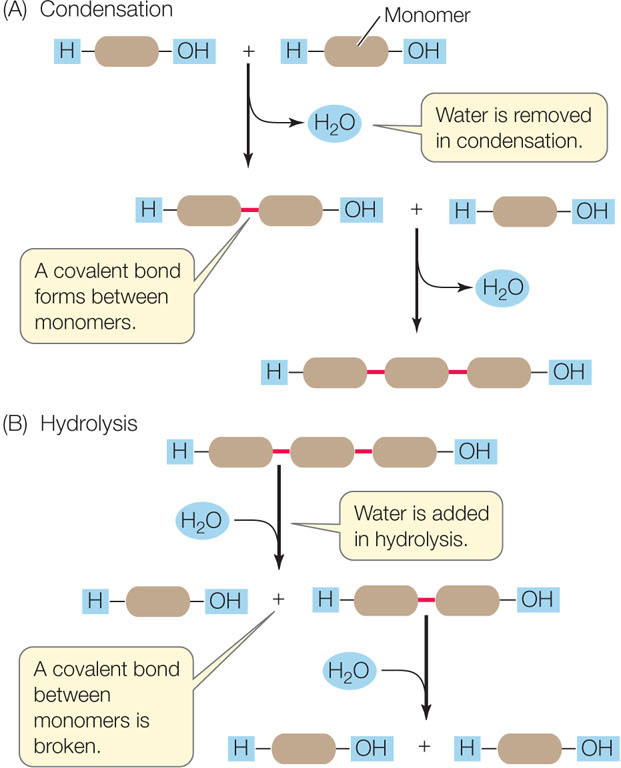
Polymers are formed and broken down by two types of reactions involving water (FIGURE 2.8):
- In condensation, the removal of water links monomers together.
- In hydrolysis, the addition of water breaks a polymer into monomers.
How the macromolecules function and interact with other molecules depends on the properties of the functional groups in their monomers.

Go to ANIMATED TUTORIAL 2.2 Macromolecules: Carbohydrates and Lipids
PoL2e.com/at2.2
CHECKpoint CONCEPT 2.2
- How do differences in electronegativity result in the unequal sharing of electrons in polar molecules?
- Some functional groups (see Figure 2.7) can either donate or accept hydrogen bonds with other molecules, acting either as a donor (like the oxygen in a water molecule) or as an acceptor (like the hydrogens in a water molecule). For each of the following, is it an H-bond donor, acceptor, both, or neither?
- Aldehyde
- Amino
- Hydroxyl
- Here is the structure of the molecule glycine:
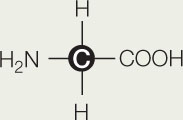
- What are the functional groups on this molecule? What is the R group to which they are attached? Is the R group hydrophilic or hydrophobic? Explain.
- Draw two glycine molecules and show how they can be linked by a condensation reaction.
- The boiling point (the temperature at which a liquid vaporizes) of water (H2O) is 100°C, whereas the boiling point of methane (CH4) is −161°C. Explain this difference in terms of hydrogen bonding between molecules.
We will begin our discussion of the molecules of life with carbohydrates, as they exemplify many of the chemical principles we have outlined so far.
27