Concept 3.2: Proteins Are Polymers with Important Structural and Metabolic Roles
Proteins are the fourth and final type of biological macromolecule we will discuss, and in terms of structural diversity and function, they are at the top of the list. Here are some of the major functions of proteins in living organisms:
- Enzymes are catalytic molecules that speed up biochemical reactions. Most enzymes are proteins (some are RNA molecules).
- Defensive proteins such as antibodies recognize and respond to substances or particles that invade the organism from the environment.
- Hormonal and regulatory proteins such as insulin control physiological processes.
- Receptor proteins receive and respond to molecular signals from inside and outside the organism.
- Storage proteins store chemical building blocks—amino acids—for later use.
- Structural proteins such as collagen provide physical stability and enable movement.
- Transport proteins such as hemoglobin carry substances within the organism.
- Genetic regulatory proteins (transcription factors) regulate when, how, and to what extent a gene is expressed.
Clearly, the biochemistry of proteins warrants our attention!
Amino acids are the building blocks of proteins
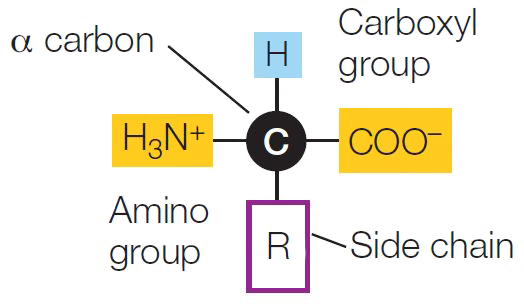
As we noted in Chapter 2, proteins are polymers made up of monomers called amino acids. As their name suggests, the amino acids all contain two functional groups: the nitrogen-containing amino group and the (acidic) carboxyl group.
43
The amino and carboxyl groups shown in the diagram are charged. How does this happen? Under the conditions that exist in most living systems, the carboxyl group releases an H+ (a cation), leaving the rest of the group as an anion:
—COOH → —COO − + H+
From your studies of chemistry, you may recognize the carboxyl group as an acid. Conversely, under the same conditions the amino group tends to form a bond with H+:
—NH2 + H+ → —NH3+
Your chemistry knowledge should tell you that the amino group is a base.
The central carbon atom of an amino acid—the α (alpha) carbon—has four available electrons for covalent bonding. In all amino acids, two of the electrons are occupied by the two functional groups noted above, and a third is occupied by a hydrogen atom. The fourth bonding electron is shared with a group that differs in each amino acid. This is often referred to as the R group, or side chain, and is designated by the letter R. Each amino acid is identified by its R group.
Go to ACTIVITY 3.3 Features of Amino Acids
PoL2e.com/ac3.3
There are hundreds of amino acids known in nature, and many of these occur in plants. But only 20 amino acids (listed in TABLE 3.2) occur extensively in the proteins of all organisms. These 20 amino acids can be grouped according to the properties conferred by their side chains (R groups):
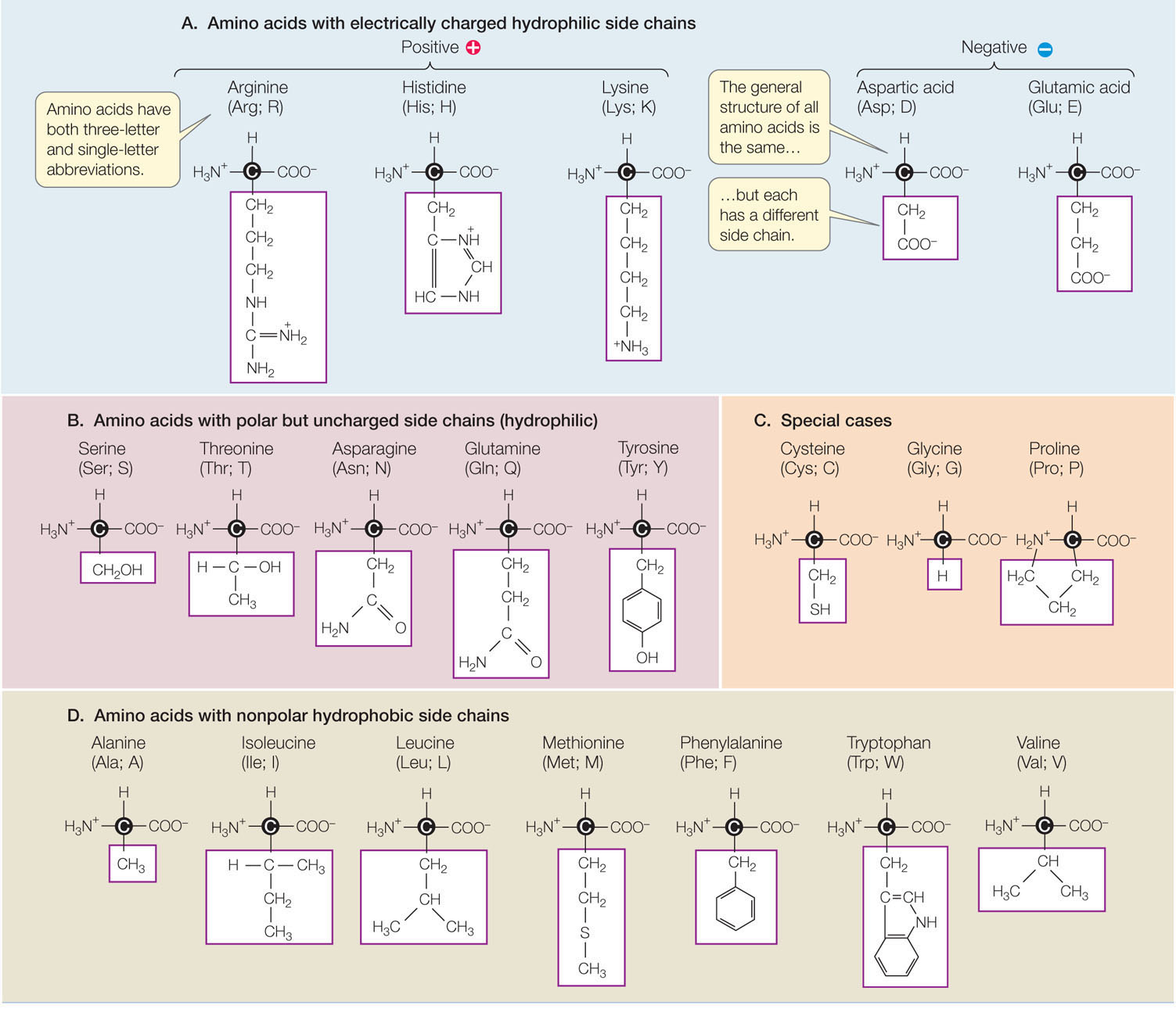
- Five amino acids have electrically charged side chains (+1 or −1), attract water (are hydrophilic), and attract oppositely charged ions of all sorts.
- Five amino acids have polar side chains (δ+, δ−) and tend to form hydrogen bonds with water and other polar or charged substances. These amino acids are also hydrophilic.
- Seven amino acids have side chains that are nonpolar hydrocarbons or very slightly modified hydrocarbons. In the watery environment of the cell, these hydrophobic side chains may cluster together in the interior of the protein.
Three amino acids—glycine, proline, and cysteine—are special cases, although the side chains of the former two generally are hydrophobic:
- The glycine side chain consists of a single hydrogen atom and is small enough to fit into tight corners in the interior of a protein molecule, where a larger side chain could not fit.
- Proline possesses a modified amino group that lacks a hydrogen atom and instead forms a covalent bond with the hydrocarbon side chain, resulting in a ring structure. This limits both its hydrogen-bonding ability and its ability to rotate. Thus proline often functions to stabilize bends or loops in proteins.
- The cysteine side chain, which has a terminal —SH group, can react with another cysteine side chain to form a covalent bond called a disulfide bridge, or disulfide bond (—S—S—). Disulfide bridges help determine how a protein molecule folds.
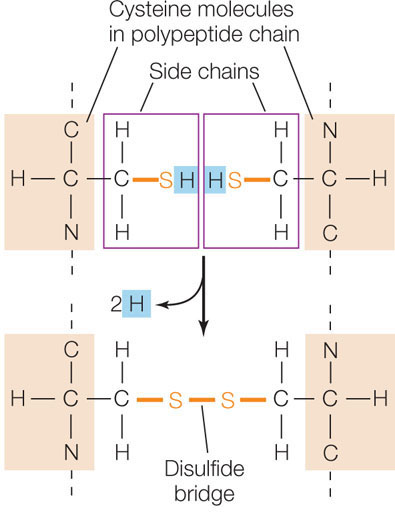
In the reaction shown above, the two —SH groups each lose a hydrogen atom (a proton and an electron) and become oxidized. The —SH group, which carries the extra electron and proton, is in its reduced state.
LINK
In addition to their role in protein structure, oxidation and reduction reactions are important in cellular metabolism; see Concept 6.1
Amino acids are linked together by peptide bonds
Amino acids can form short polymers of 20 or fewer amino acids, called oligopeptides or simply peptides. These include some hormones and other molecules involved in signaling from one part of an organism to another. Even with their relatively short chains of amino acids, oligopeptides have distinctive three-dimensional structures.
More common are the longer polymers called polypeptides, each with a unique sequence of amino acids. A functional protein may be made up of one or more polypeptides. Proteins range in size from small ones such as insulin, which has 51 amino acids, to huge molecules such as the muscle protein titin, with 34,350 amino acids.
Like nucleic acids, oligopeptides and polypeptides form via the sequential addition of new amino acids to the ends of existing chains. The amino group of the new amino acid reacts with the carboxyl group of the amino acid at the end of the chain. This condensation reaction forms a peptide bond (FIGURE 3.6). Note that there is directionality here, just as with the nucleic acids. In this case, polymerization takes place in the amino to carboxyl direction.
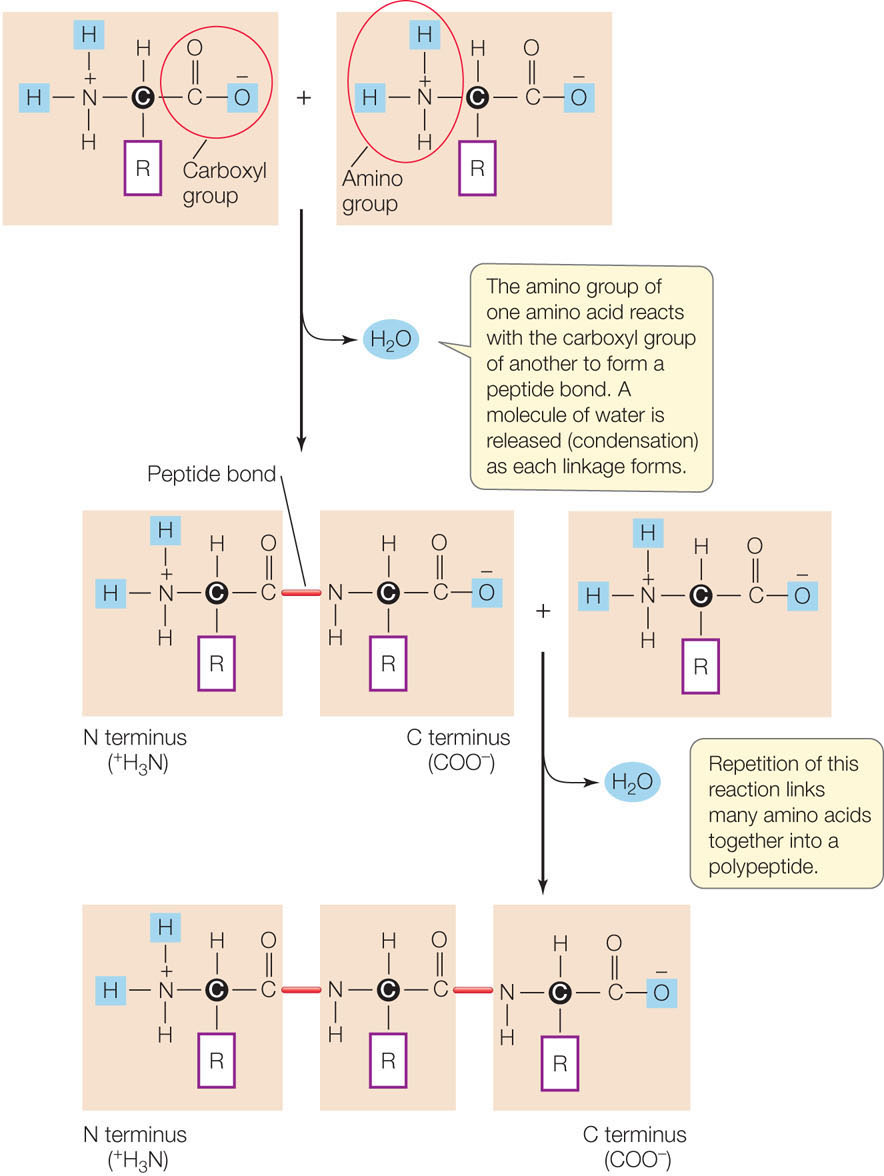
The precise sequence of amino acids in a polypeptide chain is the primary structure of a protein. Scientists have determined the primary structures of many proteins. The singleletter abbreviations for amino acids (see Table 3.2) are used to record the amino acid sequences of proteins. Here, for example, are the first 20 amino acids (out of a total of 1,827) in the human protein sucrase:
MARKKFSGLEISLIVLFVIV
The theoretical number of different proteins is enormous. Since there are 20 different amino acids, there could be 20 × 20 = 400 distinct dipeptides (two linked amino acids) and 20 × 20 × 20 = 8,000 different tripeptides (three linked amino acids). So for even a small polypeptide of 100 amino acids there are 20100 possible sequences, each with its own distinctive primary structure. How large is the number 20100? Physicists tell us there aren’t that many electrons in the entire universe.
Higher-level protein structure is determined by primary structure
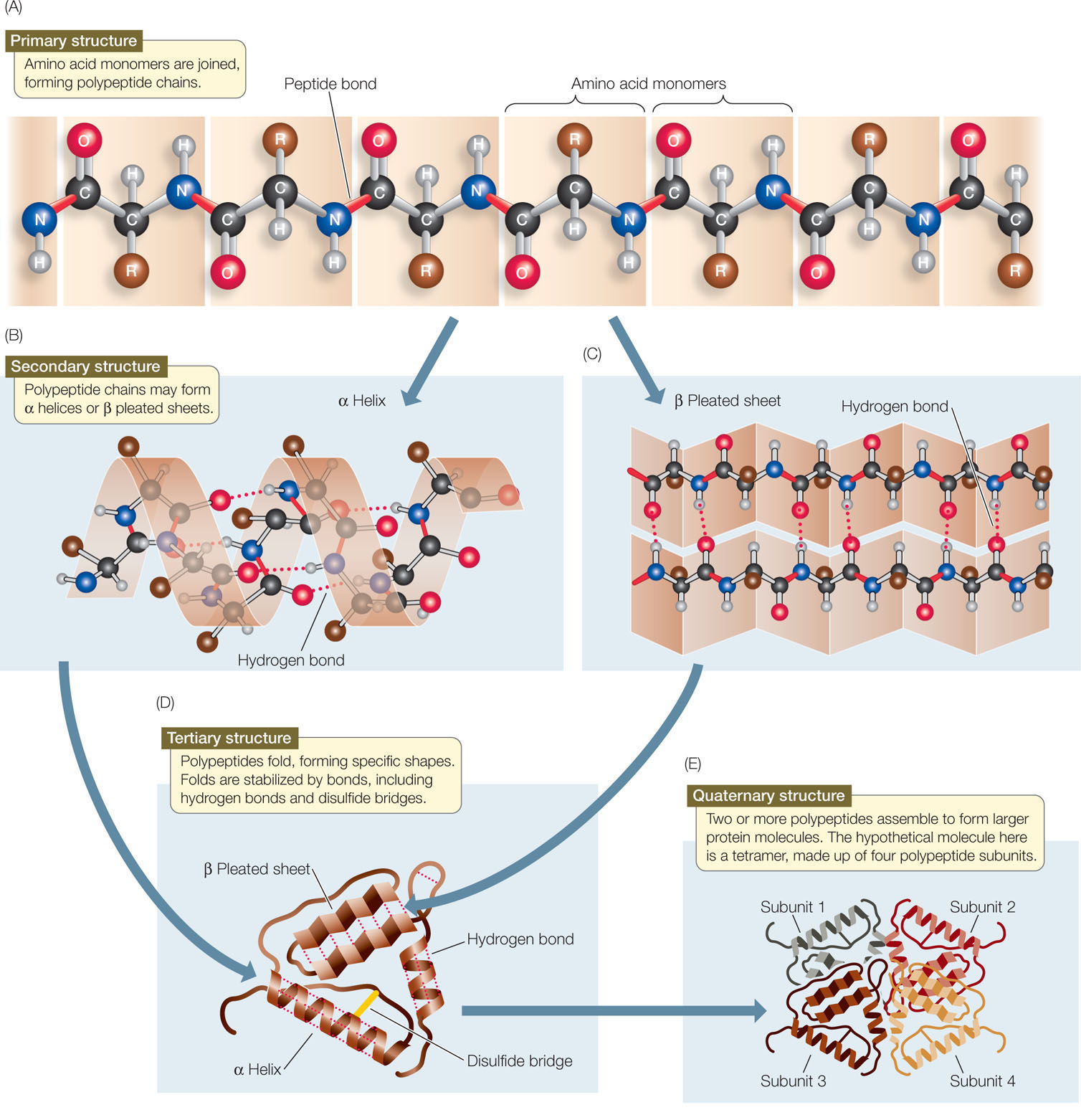
The primary structure of a protein is established by covalent bonds, but higher levels of structure are determined largely by weaker forces, including hydrogen bonds and hydrophobic and hydrophilic interactions. Follow FIGURE 3.7 as we describe how a protein chain becomes a three-dimensional structure.
Secondary Structure
A protein’s secondary structure consists of regular, repeated spatial patterns in different regions of a polypeptide chain. There are two basic types of secondary structure, both determined by hydrogen bonding between the amino acids that make up the primary structure:
- The α (alpha) helix is a right-handed coil that turns in the same direction as a standard wood screw (see Figure 3.7B). The R groups extend outward from the peptide backbone of the helix. The coiling results from hydrogen bonds that form between the N—H group on one amino acid and the C
 O group on another within the same turn of the helix.
O group on another within the same turn of the helix. - The β (beta) pleated sheet is formed from two or more sequences of amino acids that are extended and aligned. The sheet is stabilized by hydrogen bonds between the N—H groups and the C
 O groups on the two chains (see Figure 3.7C). A β pleated sheet may form between separate polypeptide chains or between different regions of a single polypeptide chain that is bent back on itself. Many proteins contain both α helices and β pleated sheets in different regions of the same polypeptide chain.
O groups on the two chains (see Figure 3.7C). A β pleated sheet may form between separate polypeptide chains or between different regions of a single polypeptide chain that is bent back on itself. Many proteins contain both α helices and β pleated sheets in different regions of the same polypeptide chain.
Tertiary Structure
In many proteins, the polypeptide chain is bent at specific sites and then folded back and forth, resulting in tertiary structure (see Figure 3.7D). Tertiary structure results in the polypeptide’s definitive three-dimensional shape, including a buried interior as well as a surface that is exposed to the environment. The protein’s exposed outer surfaces present functional groups capable of interacting with other molecules in the cell. These molecules might be other proteins or smaller chemical reactants (as in enzymes; see below).
Whereas hydrogen bonding between the N—H and C O groups within and between chains is responsible for a protein’s secondary structure, it is the interactions between R groups—the amino acid side chains—that determine tertiary structure (FIGURE 3.8):
O groups within and between chains is responsible for a protein’s secondary structure, it is the interactions between R groups—the amino acid side chains—that determine tertiary structure (FIGURE 3.8):
- Covalent disulfide bridges can form between specific cysteine side chains, holding a folded polypeptide together.
- Hydrogen bonds between side chains also stabilize folds in proteins.
- Hydrophobic side chains can aggregate together in the interior of a protein, away from water, folding the polypeptide in the process.
- van der Waals interactions can stabilize close associations between hydrophobic side chains.
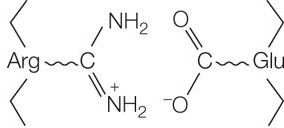
- Ionic interactions can form between positively and negatively charged side chains, forming “salt bridges” between amino acids. Ionic interactions can also be buried deep within a protein, away from water. These interactions occur between positively and negatively charged amino acids, for example arginine (which has a positively charged R group) and glutamic acid (which has a negatively charged R group):
LINK
To review the strong and weak interactions that can occur between atoms, see Concept 2.2
A complete description of a protein’s tertiary structure would specify the location of every atom in the molecule in three-dimensional space, relative to all the other atoms. Many such descriptions are available, including one for the human protein sucrase (FIGURE 3.9).
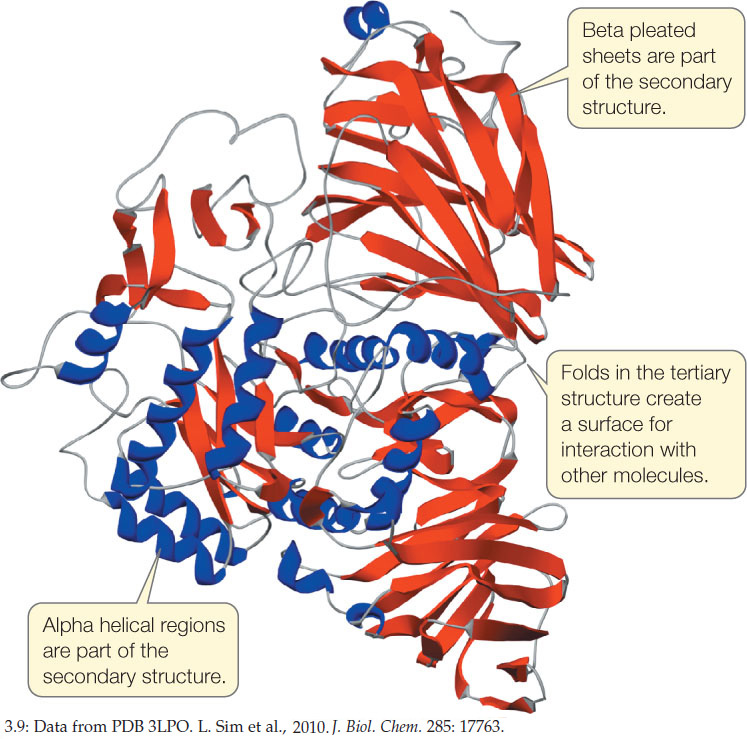
Remember that both secondary and tertiary structure derive from primary structure. If a protein is heated slowly, the heat energy will disrupt only the weaker interactions, causing the secondary and tertiary structure to break down. The protein is then said to be denatured. Chemical treatments can also be used to denature proteins. In many cases a denatured protein can return to its normal tertiary structure when it cools or the denaturing chemicals are removed, demonstrating that all the information needed to specify the protein’s unique shape is contained in its primary structure. This fact was first shown by biochemist Christian Anfinsen for the protein ribonuclease (FIGURE 3.10).
46
Investigation
HYPOTHESIS
Under controlled conditions that simulate the normal cellular environment, a denatured protein can refold into a functional three-dimensional structure.
METHOD
Chemically denature a functional ribonuclease, so that only its primary structure (i.e., an unfolded polypeptide chain) remains.
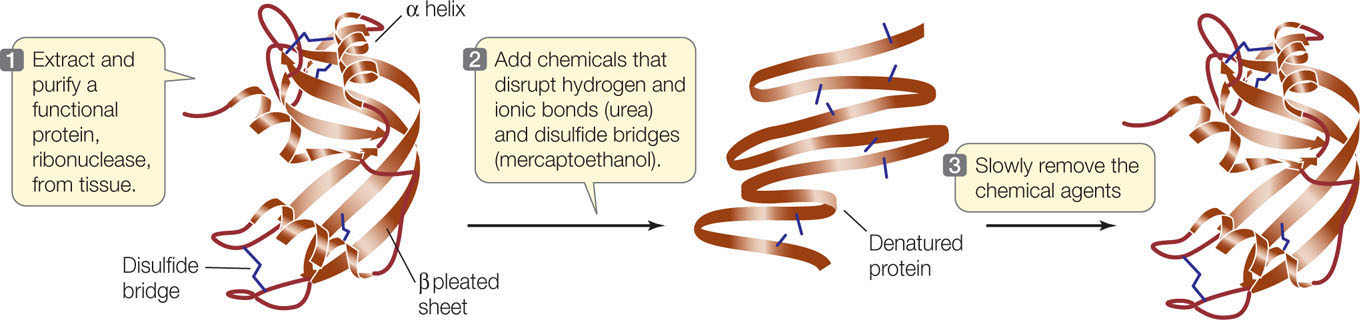
RESULTS
When the disruptive agents are removed, three-dimensional structure is restored and the protein once again is functional.
CONCLUSION
In normal cellular conditions, the primary structure of a protein specifies how it folds into a functional, three-dimensional structure.
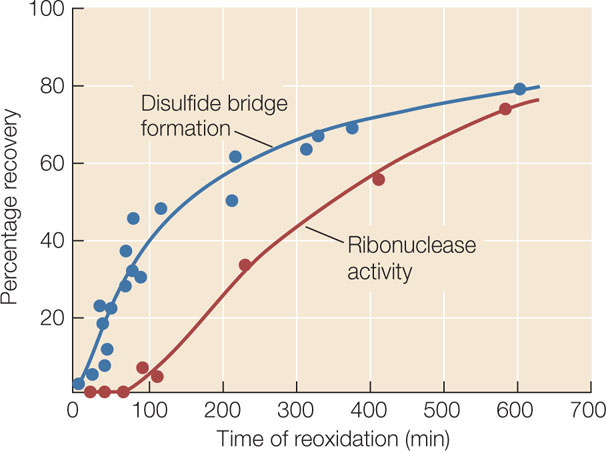
ANALYZE THE DATA
Initially, disulfide bridges (S—S) in ribonuclease were eliminated because the sulfur atoms in cysteine were reduced (—SH). At time 0, reoxidation began and at various times, the amount of disulfide bridge re-formation (blue circles) and the function of ribonuclease (enzyme activity; red circles) were measured by chemical methods. Here are the data:
- At what time did disulfide bridges begin to form?
- At what time did enzyme activity begin to appear?
- Explain the difference between your answers for the times of (A) and (B).
Go to LaunchPad for discussion and relevant links for all INVESTIGATION figures.
aC. B. Anfinsen et al. 1961. Proceedings of the National Academy of Sciences USA. 47: 1309–1314.
Quaternary Structure
Many functional proteins contain two or more polypeptide chains, called subunits, each folded into its own unique tertiary structure. The protein’s quaternary structure results from the ways in which these subunits bind together and interact (see Figure 3.7E). Hemoglobin is an example of a protein with multiple subunits:
47
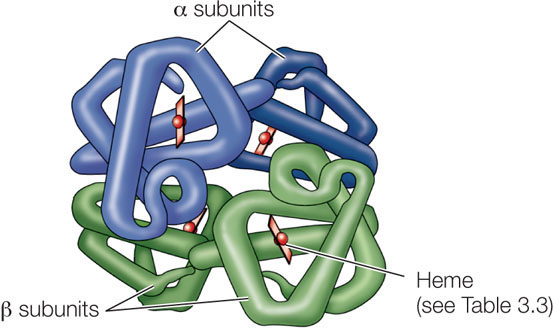
Hydrophobic interactions, hydrogen bonds, and ionic interactions all help hold the four subunits together to form a hemoglobin macromolecule. The weak nature of these forces permits small changes in the quaternary structure to aid the protein’s function—which is to carry oxygen in red blood cells. As hemoglobin binds one O2 molecule, the four subunits shift their relative positions slightly, changing the quaternary structure. Ionic interactions are broken, exposing buried side chains that enhance the binding of additional O2 molecules. The quaternary structure changes again when hemoglobin releases its O2 molecules to the cells of the body.
Protein structure can change
The environment that surrounds a protein, as well as interactions with other molecules, can change protein structure.
Environment
Various conditions can alter the weak, noncovalent interactions that hold proteins together in their secondary, tertiary, and quaternary structures:
- Increases in temperature cause more rapid molecular movements and thus can break hydrogen bonds and hydrophobic interactions.
- Alterations in the concentration of H+ (pH) in the solution surrounding the protein can change the patterns of ionization of the exposed carboxyl and amino groups. This can disrupt the patterns of ionic attractions and repulsions.
- High concentrations of polar substances such as urea can disrupt the hydrogen bonding that is crucial to protein structure.
- Nonpolar substances may also denature a protein in cases where hydrophobic groups are essential for maintaining the protein’s structure.

Go to MEDIA CLIP 3.1 Protein Structures in 3D
PoL2e.com/mc3.1
Denaturation can be irreversible when amino acids that were buried in the interior of the protein become exposed at the surface, or vice versa, causing a new structure to form, or causing different molecules to bind to the protein. Boiling an egg denatures its proteins and is, as you know, not reversible.
Molecular Interactions
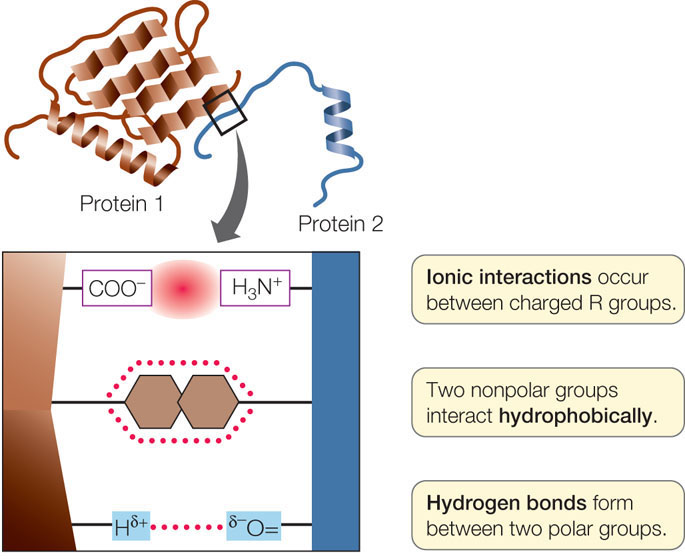
Proteins do not exist in isolation. Within a living organism, a protein may interact with other proteins, other kinds of macromolecules, or a variety of smaller molecules. These interactions are reminiscent of the interactions that make up quaternary structure (see above). If a polypeptide comes into contact with another molecule, R groups on its surface may form weak interactions (such as hydrogen bonds or ionic interactions) with groups on the surface of the other molecule. This may disrupt some of the interactions between R groups within the polypeptide, causing it to undergo a change in shape (FIGURE 3.11A).
48
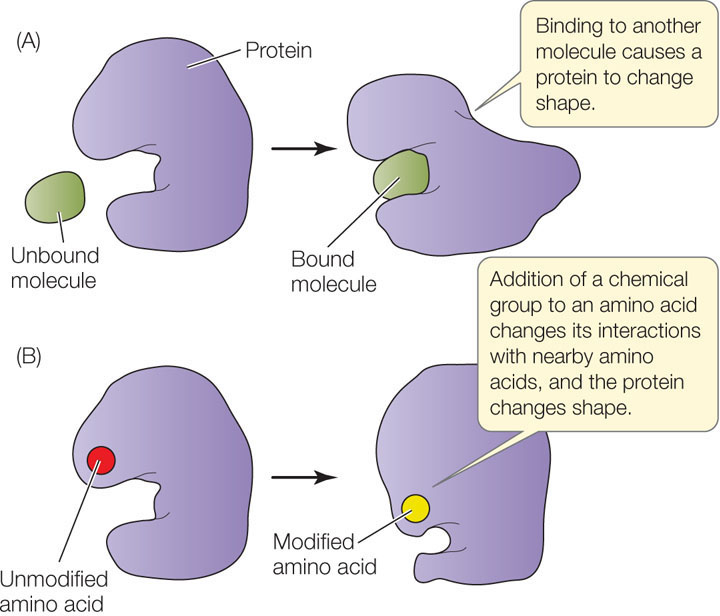
The structure of a protein can also be modified by the covalent bonding of a chemical group to the side chain of one or more of its amino acids. The chemical modification of just one amino acid can alter the shape and function of a protein. An example is the addition of a charged phosphate group to a relatively nonpolar R group. This can cause the amino acid to become more hydrophilic and to move to the outer surface of the protein, altering the shape of the protein in the region near the amino acid (FIGURE 3.11B).
49
CHECKpoint CONCEPT 3.2
- Sketch the peptide bonding of the two amino acids glycine and leucine (in that order). Now add a third amino acid, alanine, in the position it would have if added within a biological system. What is the directionality of this process?
- Examine the structure of sucrase (see Figure 3.9). Where in the protein might you expect to find the following amino acids: valine, proline, glutamic acid, and threonine? Explain your answers.
- Detergents disrupt hydrophobic interactions by coating hydrophobic molecules with a molecule that has a hydrophilic surface. When hemoglobin is treated with a detergent, the four polypeptide chains separate and become random coils. Explain these observations.
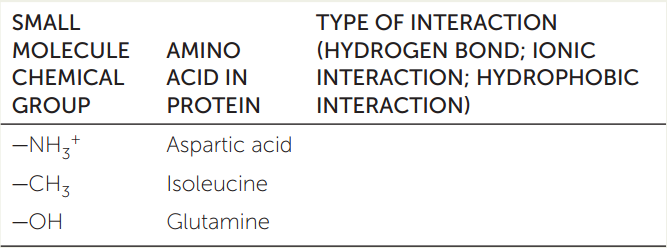
- Several small molecules interact with a protein. The chemical groups on the small molecules interact with specific amino acids as shown in the table below. Fill in the table to show the types of noncovalent interactions that occur between the small molecules and the amino acids.
We have discussed the remarkable diversity in protein structures. These structures carry functional groups (on exposed amino acid side chains) that can interact with other molecules. In the next section we will see how these interactions can result in catalysis, the speeding up of biochemical reactions.
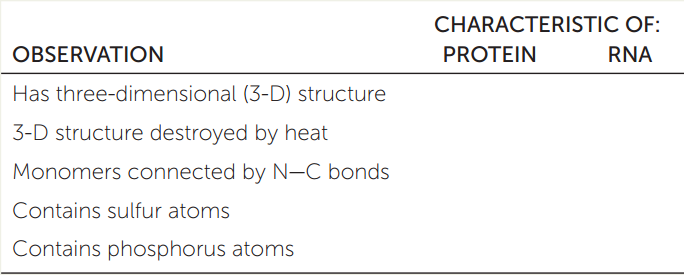
APPLY THE CONCEPT: Proteins are polymers with important structural and metabolic roles
Biological systems contain “supermolecular complexes” (for example, the ribosome; see Chapter 4), which are composed of individual molecules of RNA and protein that fit together noncovalently. These complexes can be split apart with detergents that disrupt hydrophobic interactions. Based on the concepts discussed in this chapter, fill in the table below to indicate which of the observations are characteristic of RNA, which are characteristic of protein, and which are characteristic of both. Explain your answers.