Concept 4.4: The Cytoskeleton Provides Strength and Movement
 The interior of the cell has a meshwork of protein filaments. Each type of filament is a polymer, made up of monomers that are proteins (which in turn are polymers of amino acids). This cytoskeleton fills several important roles:
The interior of the cell has a meshwork of protein filaments. Each type of filament is a polymer, made up of monomers that are proteins (which in turn are polymers of amino acids). This cytoskeleton fills several important roles:
- It supports the cell and maintains its shape.
- It holds cell organelles and other particles in position within the cell.
- It moves organelles and other particles around within the cell.
- It is involved with movements of the cytoplasm called cytoplasmic streaming.
- It interacts with extracellular structures, helping anchor the cell in place.
There are three components of the eukaryotic cytoskeleton: microfilaments (smallest diameter), intermediate filaments, and microtubules (largest diameter). These filaments have very different functions.
Microfilaments are made of actin
Microfilaments (FIGURE 4.10A) are usually in bundles. Each filament is about 7 nm in diameter and up to several micrometers long. Microfilaments have two major roles:
- They help the entire cell or parts of the cell to move.
- They determine and stabilize cell shape.
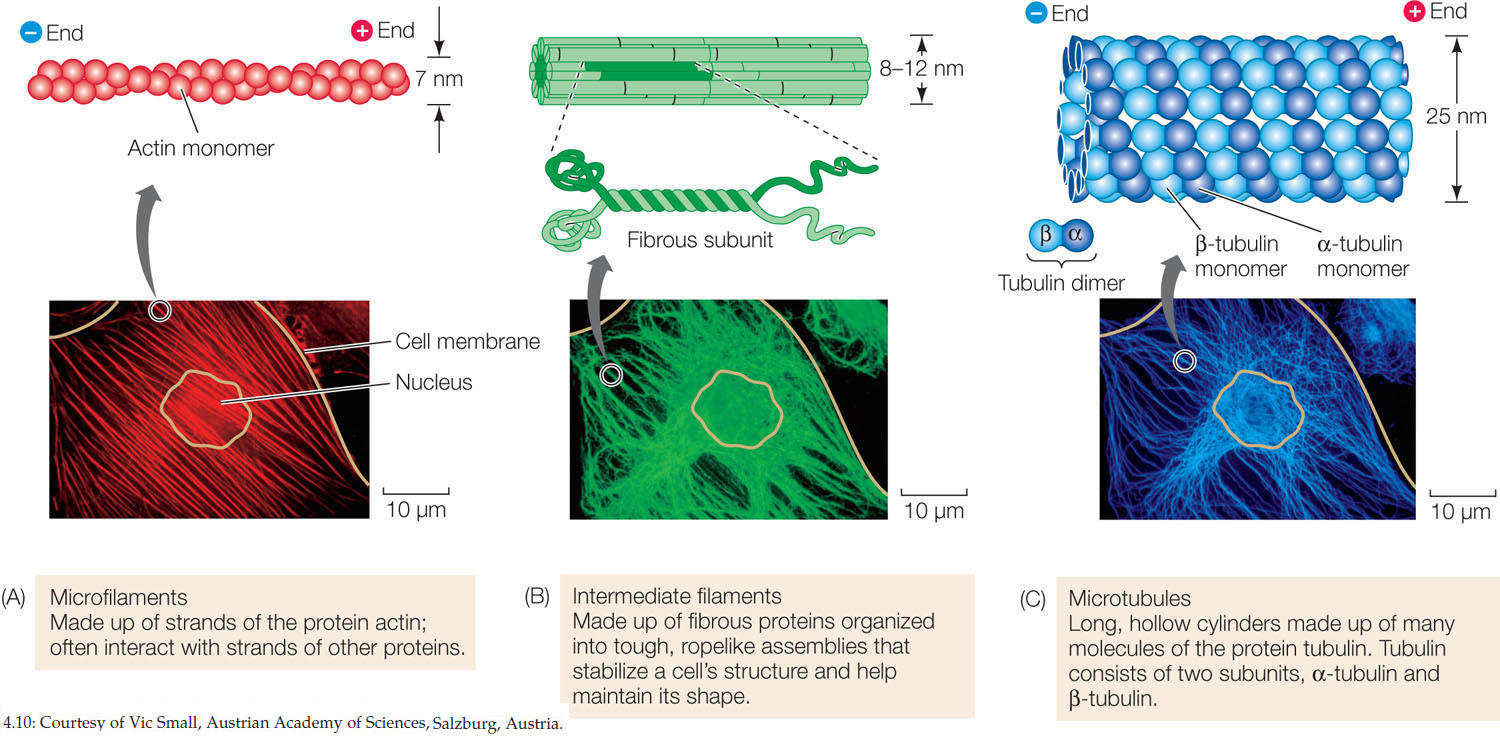
Microfilaments are assembled from actin monomers that attach to the filament at one end (the “plus end”) and detach at the other (the “minus end”). In an intact filament, assembly and detachment are in equilibrium. But sometimes the filaments can shorten (more detachment) or lengthen (more assembly):
Actin polymer (filament) ⇌ actin monomers
This property of dynamic instability is a hallmark of the cytoskeleton. Portions of it can be made and broken down rather quickly, depending on cell function. Actin-associated proteins work at both ends of the filament to catalyze assembly and disassembly.
In the muscle cells of animals, actin filaments are associated with the motor protein myosin, and the interactions of these two proteins account for the contraction of muscles. A motor protein (or molecular motor) is any protein that causes movement within a cell. In non-muscle cells, actin filaments are associated with localized changes in cell shape. For example, microfilaments are involved in the flowing movement of the cytoplasm called cytoplasmic streaming, and in the “pinching” contractions that divide an animal cell into two daughter cells. Microfilaments are also involved in the formation of cellular extensions called pseudopodia (pseudo. “false”; podia. “feet”) that enable some cells (such as Amoeba. see Figure 4.14) to move.
Intermediate filaments are diverse and stable
There are at least 50 different kinds of intermediate filaments (FIGURE 4.10B), many of them specific to just a few cell types. They generally fall into six molecular classes based on amino acid sequence. One of these classes consists of the fibrous keratin proteins, which are also found in hair and fingernails. The intermediate filaments are tough, ropelike protein assemblages 8–12 nm in diameter. Intermediate filaments are more permanent than the other two types of filaments and do not show dynamic instability. Intermediate filaments have two major structural functions:
- They anchor cell structures in place. In some cells, intermediate filaments radiate from the nuclear envelope and help maintain the positions of the nucleus and other organelles in the cell.
- They resist tension. For example, they maintain rigidity in body surface tissues by extending through the cytoplasm and connecting specialized membrane structures called desmosomes (see Figure 4.18).
Microtubules are the thickest elements of the cytoskeleton
Microtubules (FIGURE 4.10C) are long, hollow, unbranched cylinders about 25 nm in diameter and up to several micrometers long. Microtubules have two roles:
- They form a rigid internal skeleton for some cells or cell regions.
- They act as a framework along which motor proteins can move structures within the cell.
Microtubules are assembled from dimers of the protein tubulin. The dimers consist of one molecule each of α-tubulin and β-tubulin. Thirteen chains of tubulin dimers surround the hollow microtubule. Like microfilaments, microtubules show dynamic instability, with plus and minus ends and associated proteins.
Microtubule ⇌ tubulin dimers
Tubulin polymerization results in a rigid structure, and tubulin depolymerization leads to its collapse. Microtubules often form an interior skeleton for projections that come out of the cell membrane, such as cilia and flagella (see below).
LINK
Microtubules are important components of the spindle, which separates chromosomes during cell division; see Concept 7.2
Cilia and flagella provide mobility
Microtubules internally line movable cell appendages: the cilia (singular cilium; FIGURE 4.11) and the flagella (see Concept 4.2). Many eukaryotic cells have one or both of these appendages, which are projections of the cell membrane lined with microtubules and their associated proteins:
- Cilia are only 0.25 μm in length. They are present by the hundreds and move stiffly either to propel a cell (for example, in protists) or to move fluid over a stationary cell (as in the human respiratory system).
- Flagella are much longer—100 to 200 μm—and occur singly or in pairs. They can push or pull the cell through its aqueous environment.
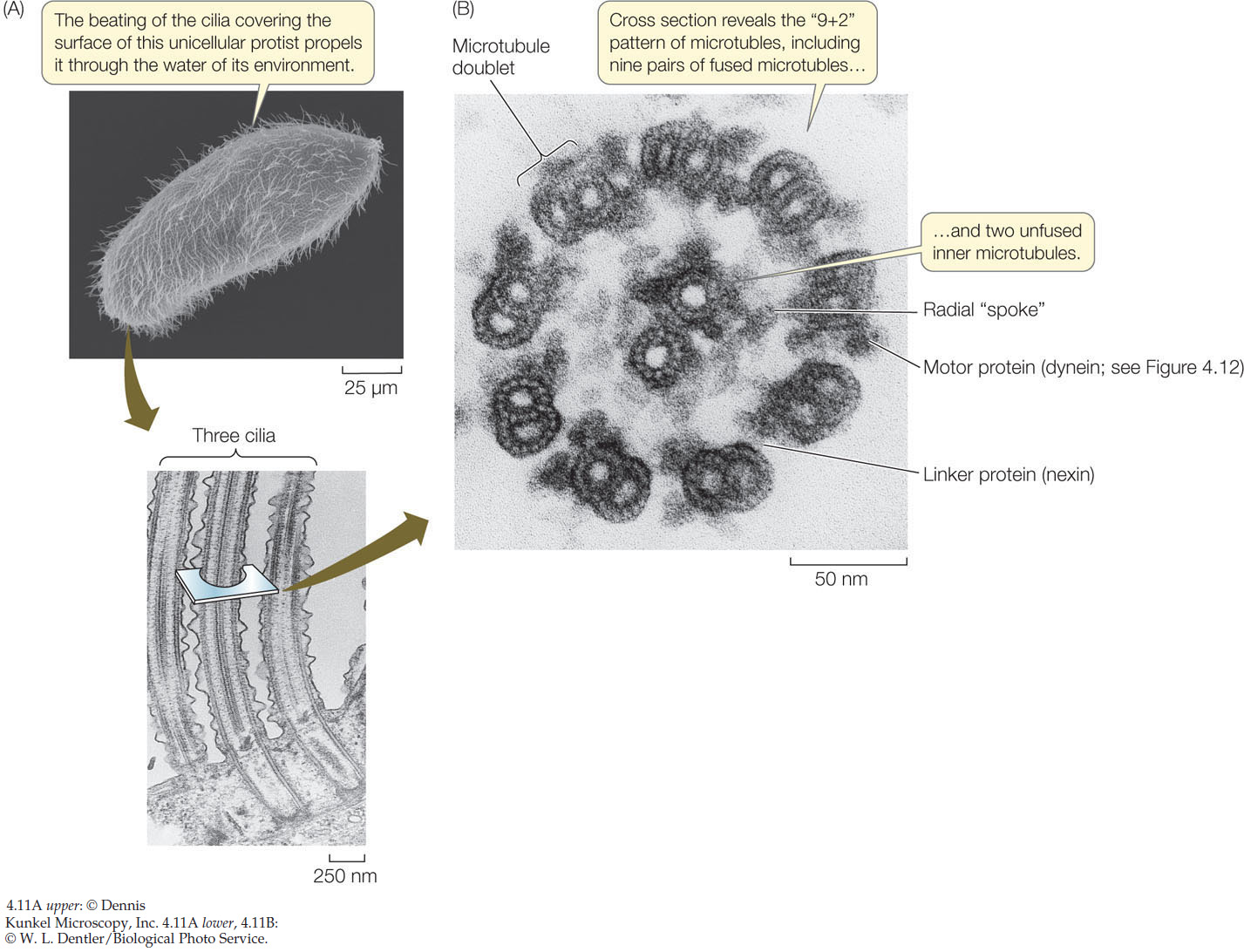
The microtubules that line cilia and flagella do more than just make them rigid. Microtubules and their associated proteins are responsible for the movement of these organelles by bending.
In cross section, a typical cilium or eukaryotic flagellum is surrounded by the cell membrane and contains a “9 + 2” array of microtubules. As Figure 4.11B shows, nine fused pairs of microtubules—called doublets—form an outer cylinder, and one pair of unfused microtubules runs up the center. Each doublet is connected to the center of the structure by a radial spoke. This structure is essential to the bending motions of both cilia and flagella. How does this bending occur?
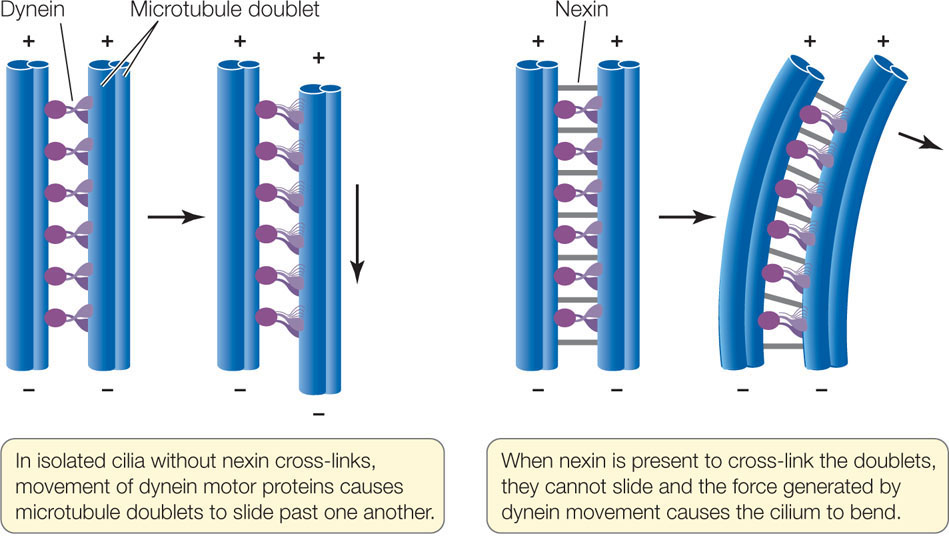
The motion of cilia and flagella results from the sliding of the microtubule doublets past one another. This sliding is driven by a motor protein called dynein, which can change its three-dimensional shape. (All motor proteins work by undergoing reversible shape changes powered by energy from ATP hydrolysis.) Dynein molecules bind between two neighboring microtubule doublets. As the dynein molecules change shape, they move the doublets past one another (FIGURE 4.12). Another protein, nexin, can cross-link the doublets and prevent them from sliding past one another; in this case, the cilium bends.
75
Other motor proteins, including kinesin, carry protein-laden vesicles or other organelles from one part of the cell to another (FIGURE 4.13). These proteins bind to the organelle and “walk” it along a microtubule by a repeated series of shape changes. A slightly different form of dynein from the one that moves cilia also performs this function. Recall that microtubules are directional, with a plus end and a minus end. Dynein moves attached organelles toward the minus end, while kinesin moves them toward the plus end.
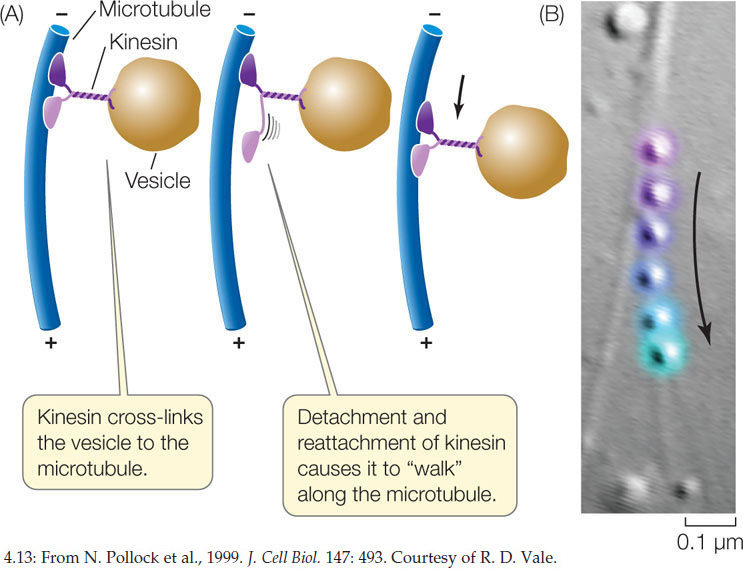
Biologists manipulate living systems to establish cause and effect
How do we know that the structural fibers of the cytoskeleton can achieve the dynamic functions described above? We can observe an individual structure under the microscope, and we can note that living cells containing that structure can perform a particular function. Such simultaneous observations suggest that the structure may carry out that function, but mere correlation does not establish cause and effect. For example, light microscopy of living cells reveals the movement of the cytoplasm within the cell. The observed presence of cytoskeletal components suggests, but does not prove. their role in this process. Scientists seek to understand the specific relationship between a structure or molecule (“A”) and a function (“B”). Two manipulative approaches are commonly used in cell biology:
76
- Inhibition: Use a drug that inhibits A, and see if B still occurs. If B does not occur, then A is probably a causative factor for B. FIGURE 4.14 shows an experiment in which an inhibitor is used to demonstrate cause and effect in the case of microfilaments and cell movement.
- Mutation: Examine a cell or organism that lacks the gene (or genes) for A, and see if B still occurs. If it does not, then A is probably a causative factor for B. You will see many examples of this experimental approach later in this book.
Investigation
HYPOTHESIS
Amoeboid cell movements are caused by microfilaments.
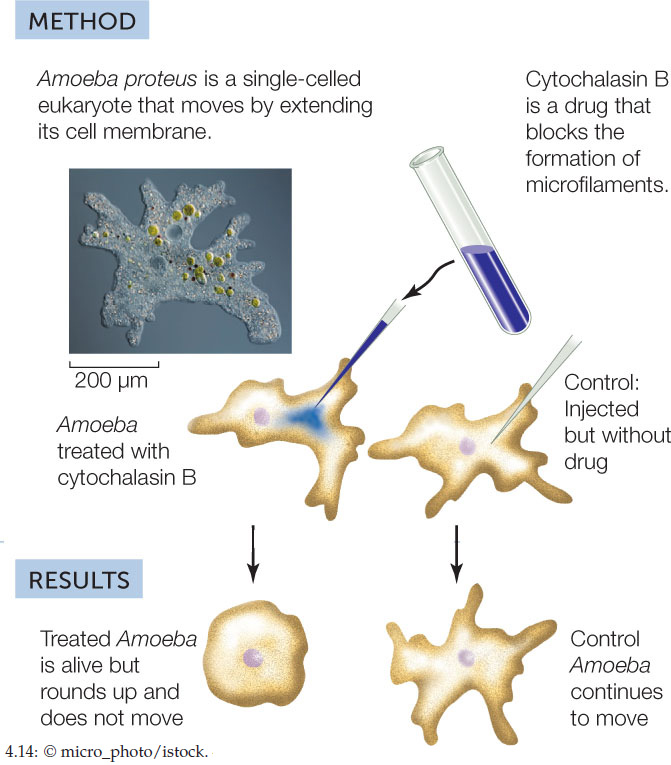
CONCLUSION
Microfilaments are essential for amoeboid cell movement.
ANALYZE THE DATA
Several important controls were done to validate the conclusions of this experiment. The experiment was repeated in the presence of cycloheximide, which inhibits new protein synthesis, and colchicine, which inhibits the polymerization of microtubules. Here are the results:

- Explain the reasoning behind each condition. Why were the controls important?
- Interpret the results of this experiment. What can you conclude about movements in Amoeba and the cytoskeleton?
Go to LaunchPad for discussion and relevant links for all INVESTIGATION figures.
aT. D. Pollard and R. R Weihing. 1974. CRC Critical Reviews in Biochemistry 2: 1–65.
CHECKpoint CONCEPT 4.4
- Make a table that compares the three major components of the cytoskeleton with regard to composition, structure, and function.
- The neuron (nerve cell) has a long extension called an axon. Molecules made in the cell’s main body must travel a long distance to reach the end of the axon. The axon is lined with microtubules. Explain how motor proteins, vesicles, and microtubules move these molecules along the axon.
- In a dividing cell, the chromosomes become very compact, and then the duplicated sets of chromosomes move along microtubules to opposite ends of the cell. How would you use an inhibitor to show that microtubules are essential for this chromosomal separation? What control treatments would you suggest?
All cells interact with their environments, and many eukaryotic cells are part of multicellular organisms and must interact with other cells. The cell membrane (which we will discuss in detail in Chapter 5) plays a crucial role in these interactions, but other structures outside that membrane are involved as well. We will now turn to these extracellular structures in animals and plants.
77