Concept 5.6: Signal Transduction Allows the Cell to Respond to Its Environment
As we mentioned in Concept 5.5, a signal may be a chemical ligand or a physical stimulus such as light or heat. Its effect is to activate a specific receptor, leading to a cellular response that is brought about by a signal transduction pathway. Typically, signaling at the cell membrane initiates a cascade (or series) of events in the cell. Proteins interact with other proteins until the final responses are achieved. Through such a cascade, an initial signal can be both amplified and distributed to cause several different responses.
Before we discuss how signals are amplified and distributed by signal transduction pathways, let’s look at some of the cellular responses that can result from cell signaling.
Cell functions change in response to environmental signals
The activation of a receptor by a signal, and the subsequent transduction and amplification of the signal, ultimately leads to changes in cell function. There are many ways in which a cell might respond, some of which we mention here:
- Opening of ion channels changes the balance of ion concentrations between the outside of the cell membrane and its interior (see Figure 5.4). As you will see in Chapter 34, this results in a change in the electrical potential across the membrane, with important consequences in nerve and muscle cells.
- Many signal transduction pathways lead to alterations in gene expression. The expression of some genes may be switched on (upregulated), whereas others may be switched off (downregulated). This alters the abundance of the proteins (often enzymes) encoded by the genes, thus changing cell function. You will see many examples that highlight the importance of gene regulation throughout this book.
- A third kind of response involves the alteration of enzyme activities. An example is the activation of specific enzymes in liver cells exposed to the hormone epinephrine, which we discuss below. An alteration in enzyme activity is a much more rapid response than one that involves a change in gene expression.
LINK
The different types of enzyme regulation are discussed in Concept 3.4
The same signal can lead to different responses in different types of cells. For example, in heart muscle cells, the hormone epinephrine activates a signal transduction cascade that results in glucose mobilization for energy and muscle contraction. However, in the smooth muscle cells that line the digestive tract, epinephrine stimulates a pathway that inhibits a target enzyme, allowing the muscle cells to relax. This increases the diameter of the blood vessels, allowing more nutrients to be carried from the digestive system to the rest of the body. Heart and digestive tract muscle cells respond differently to the same signal—epinephrine—because the signal transduction pathways stimulated by epinephrine are different in the different cell types. Let’s take a closer look at the mechanism by which cells amplify and transduce signals to bring about these responses.
Second messengers can stimulate signal transduction
Often there is a small molecule intermediary between the activated receptor and the cascade of events that ensues. In a series of clever experiments, Earl Sutherland and his colleagues at Case Western Reserve University discovered that a small, water-soluble chemical could mediate cytoplasmic events initiated by a cell membrane receptor. The researchers were investigating the activation of the liver enzyme glycogen phosphorylase by the hormone epinephrine (also called adrenaline)—the “fight-or-flight” hormone (see Concept 35.2). The enzyme is activated when an animal faces life-threatening conditions and needs energy fast for the fight-or-flight response. Glycogen phosphorylase catalyzes the breakdown of glycogen stored in the liver so that the resulting glucose molecules can be released to the blood (see Figure 30.16). The enzyme is present in the liver cell cytoplasm but is inactive in the absence of epinephrine.
The researchers found that epinephrine could activate glycogen phosphorylase in liver cells that had been broken open, but only if the entire cell contents, including cell membrane fragments, were present. Under these conditions epinephrine was bound to the cell membrane fragments, but the active phosphorylase was in the solution. The researchers hypothesized that there must be a second “messenger” that transmits the epinephrine signal (the “first messenger”) from the cell membrane to the phosphorylase in the cytoplasm. They investigated this by separating cell membrane fragments from the cytoplasmic fractions of broken liver cells and following the sequence of steps described in FIGURE 5.15. This experiment confirmed the existence of a second messenger, later identified as cyclic AMP (cAMP; FIGURE 5.16).
Investigation
HYPOTHESIS
A second messenger mediates between receptor activation at the cell membrane and enzyme activation in the cytoplasm.
METHOD
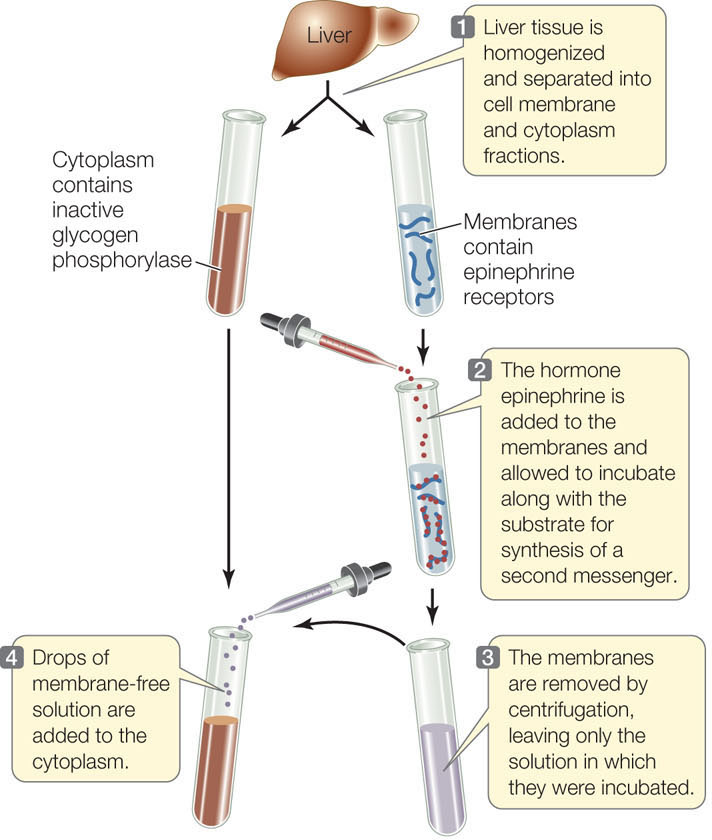
RESULTS
Active glycogen phosphorylase is present in the cytoplasm.
CONCLUSION
A soluble second messenger, produced by hormone-activated membranes, is present in the solution and activates enzymes in the cytoplasm.
ANALYZE THE DATA
The experiment was repeated under various conditions with the following results:

- What do these data show?
- Propose an experiment to show that the factor that activates the enzyme is stable on heating (and therefore probably not a protein) and give predicted data.
- Propose an experiment to show that cAMP can replace the membrane fraction and hormone treatment and give predicted data.
Go to LaunchPad for discussion and relevant links for all INVESTIGATION figures.
aT. W. Rall et al. 1957. Journal of Biological Chemistry 224: 463–475.
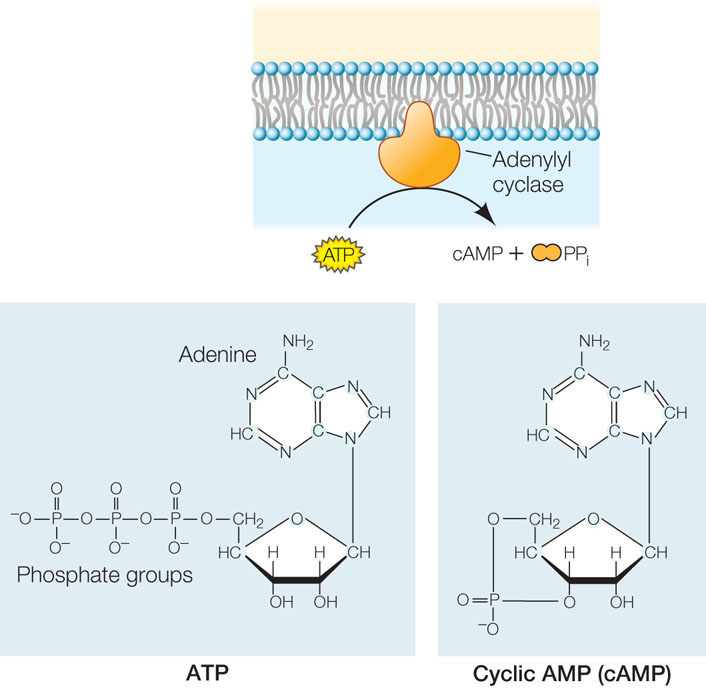
A second messenger is a small molecule that brings about later steps in a signal transduction pathway. Second messengers do not have enzymatic activity themselves; rather, they act to regulate target enzymes by binding to them noncovalently. Whereas receptor binding is highly specific, second messengers allow a cell to respond to a single event at the cell membrane with many events inside the cell—in other words, the second messenger distributes the initial signal. Second messengers also serve to amplify the signal—for example, the binding of a single epinephrine molecule leads to the production of many molecules of cAMP. In turn, cAMP activates many enzyme targets by binding to them noncovalently. In the case of epinephrine and the liver cell, glycogen phosphorylase is just one of several enzymes that are activated.
100
A signaling cascade involves enzyme regulation and signal amplification
Signal transduction pathways often involve multiple sequential steps, in which particular enzymes are either activated or inhibited by other enzymes in the pathway. For example, a protein kinase adds a phosphate group to a target protein, and this covalent change alters the protein’s conformation and activates or inhibits its function. Cyclic AMP binds noncovalently to a target protein, and this changes the protein’s shape, activating or inhibiting its function. In the case of activation, a previously inaccessible active site is exposed, and the target protein goes on to perform a new cellular role.
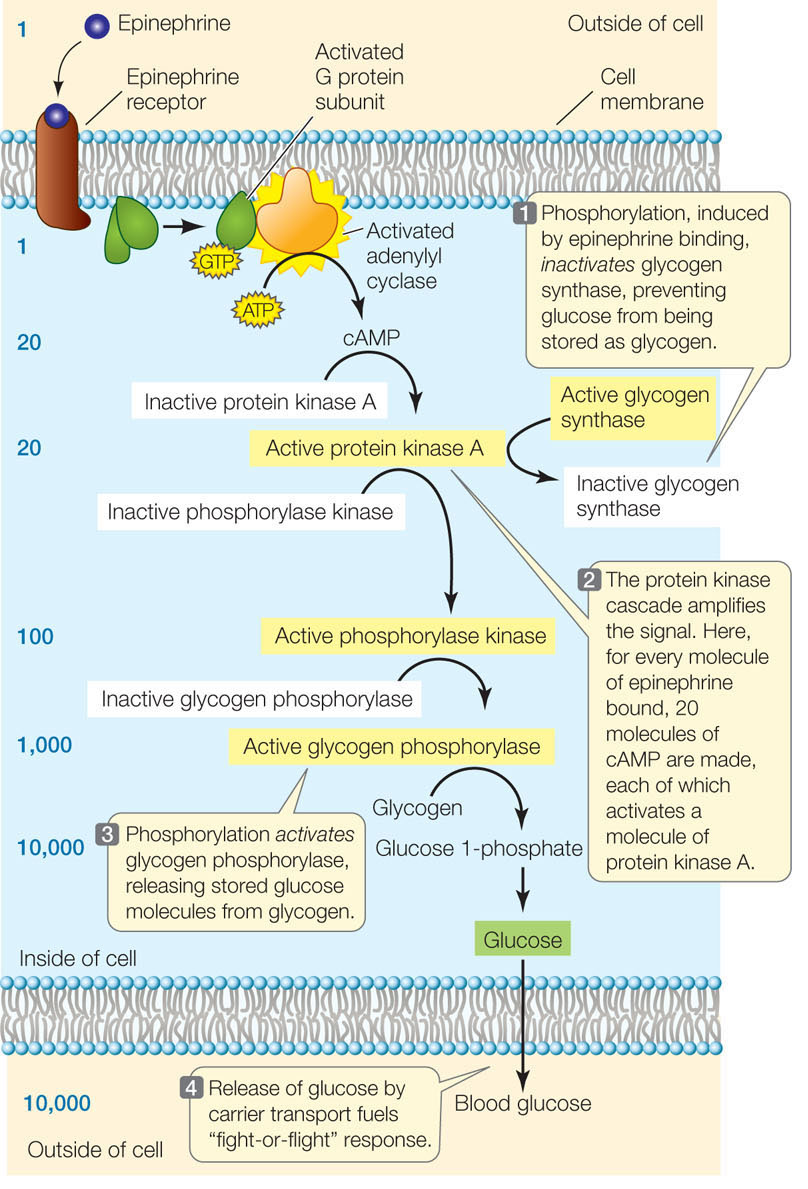
A good example of a signaling cascade is the G proteinmediated protein kinase pathway stimulated by epinephrine in liver cells (FIGURE 5.17). Binding of epinephrine to the membrane receptor results in the activation of a G protein, followed by the production of cAMP, which activates a key signaling molecule, the enzyme protein kinase A. In turn, protein kinase A phosphorylates two other enzymes, with opposite effects:
- Inhibition. Glycogen synthase, which catalyzes the joining of glucose molecules to form the energy-storing molecule glycogen, is inactivated when a phosphate group is added to it by protein kinase A. Thus the epinephrine signal prevents glucose from being stored in glycogen (see Figure 5.17, step 1).
- Activation. Phosphorylase kinase is activated when a phosphate group is added to it. It is part of a cascade of reactions that ultimately leads to the activation of glycogen phosphorylase, another key enzyme in glucose metabolism. This enzyme results in the liberation of glucose molecules from glycogen (see Figure 5.17, steps 2 and 3).

Go to ANIMATED TUTORIAL 5.6 Signal Transduction Pathway
PoL2e.com/at5.6
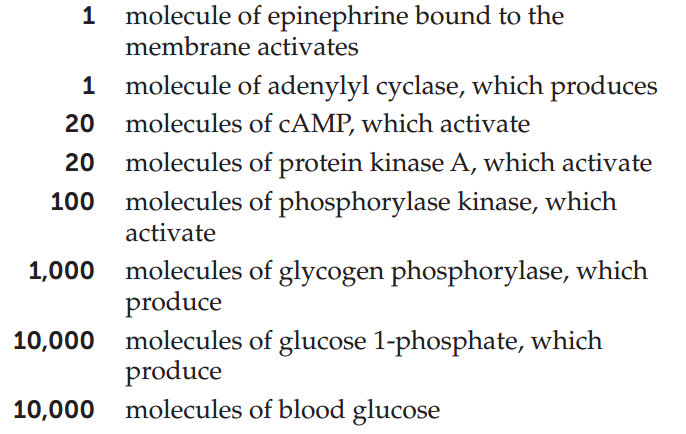
An important consequence of having multiple steps in a signal transduction cascade is that the signal is amplified with each step. The amplification of the signal in the pathway illustrated in Figure 5.17 is impressive. Each molecule of epinephrine that arrives at the cell membrane ultimately results in 10,000 molecules of blood glucose:
Signal transduction is highly regulated
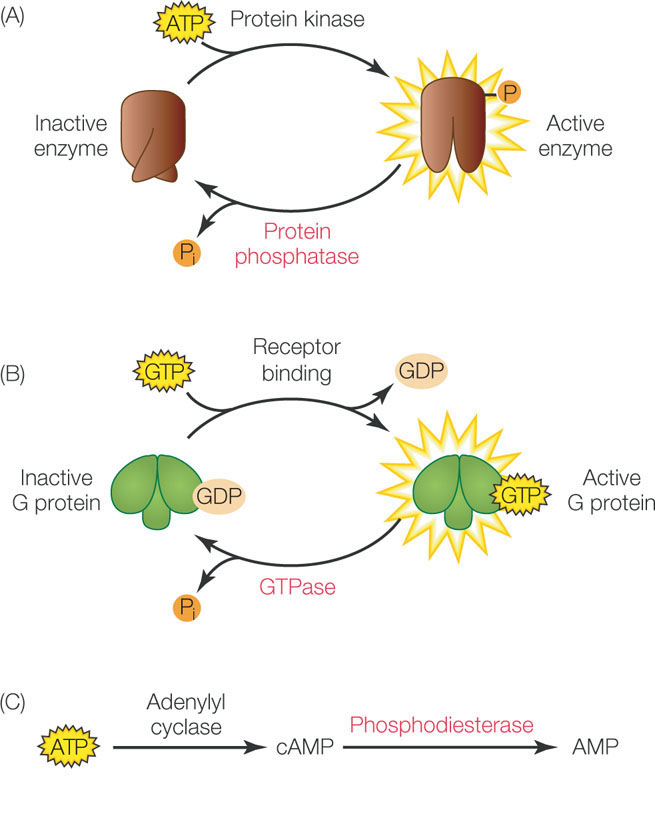
Signal transduction is a temporary event in the cell, and gets “turned off” once the cell has responded. We have already discussed the turnover of cell surface receptors by endocytosis. In addition, there are enzymes that convert signal transduction molecules back to their inactive precursors. For example, protein phosphatases remove phosphate groups from target proteins, thus reversing the effects of protein kinases (FIGURE 5.18A). G proteins have GTPase activity, which removes a phosphate group from GTP, converting it to GDP (FIGURE 5.18B). Cyclic AMP is converted back to AMP by the enzyme phosphodiesterase (FIGURE 5.18C). The balance between the activities of these regulating enzymes and the signaling enzymes themselves is what determines the ultimate cellular response to a signal. Cells can alter this balance in several ways, including:
102
- Synthesis or breakdown of the enzymes involved
- Activation or inhibition of the enzymes by other molecules (see Concept 3.4)
A great deal has been learned about signal transduction pathways and cellular responses in the past two decades, and there is still much to learn. As biologists tease apart specific pathways, they find that many of them are interconnected: one pathway may be switched on by a particular signal or molecule, and another may be switched off. In this chapter we have concentrated on signaling pathways that occur in animal cells. However, signal transduction pathways are important in the functioning of all living organisms.
CHECKpoint CONCEPT 5.6
- Compare “first messengers” (e.g., hormones) with “second messengers” (e.g., cAMP) with regard to their chemical nature, where and when they are made, and their activity.
- Outline the steps in the amplification of signaling by epinephrine, resulting in the release of glucose to the bloodstream. At each step, is the amplification due to a covalent or noncovalent interaction?
- What would happen to a liver cell exposed to epinephrine and at the same time to a drug that inhibits protein kinase A? To epinephrine and to a drug that inhibits the hydrolysis of GTP? (Assume that both these drugs are able to cross the cell membrane.)
- The disease cholera is caused by a toxin released from the bacterium Vibrio cholerae. Cholera toxin causes continuous activation of a G protein at the cell membrane of cells lining the intestine. This in turn results in continuous activation of adenylyl cyclase. As a result, there is continuous release of Na+ from the intestine, followed by massive outflow of water, resulting in severe diarrhea, dehydration, and if untreated, death. How does cholera toxin work on the second messenger system, and what is the normal role of that second messenger in the intestine cell membrane?
Question 5.2
What role does the cell membrane play in the body’s response to caffeine?
ANSWER Caffeine has many effects on the body, but the most noticeable is that it keeps us awake. The caffeine molecule is somewhat large and polar, and it is unlikely to diffuse through the nonpolar lipids of the cell membrane (Concept 5.2). Instead, it binds to receptors on the surfaces of nerve cells in the brain (Concept 5.5).
The nucleoside adenosine (adenine attached to a five-carbon sugar) accumulates in the brain when a person is under stress or has prolonged mental activity. When it binds to a specific receptor in the brain, adenosine sets in motion a signal transduction pathway (Concept 5.6) that results in reduced brain activity, which usually means drowsiness. This membrane-associated signaling by adenosine has evolved as a protective mechanism against the adverse effects of stress.
Caffeine has a three-dimensional structure similar to that of adenosine and is able to bind to the adenosine receptor (FIGURE 5.19). Because its binding does not activate the receptor, caffeine functions as an antagonist of adenosine signaling, with the result that the brain stays active and the person remains alert.

When we discussed the interaction between a ligand and its receptor, we noted that this is a reversible, noncovalent interaction. In time, after drinking coffee or tea, the caffeine molecules come off the adenosine receptors in the brain, allowing adenosine to bind once again. Otherwise, coffee drinkers might never get to sleep!
103
In addition to competing with adenosine for a membrane receptor, caffeine blocks the enzyme cAMP phosphodiesterase. This enzyme acts in signal transduction (Concept 5.6) to break down the second messenger cAMP. Looking at the signal transduction pathway in Figure 5.17, can you explain how caffeine augments the fight-or-flight response, which includes an increase in blood sugar and increased heartbeat?