Concept 8.4: Prokaryotes Can Exchange Genetic Material
As described in Concept 4.2, prokaryotic cells lack nuclei; they contain their genetic material mostly as single chromosomes in central regions of their cells. Prokaryotes reproduce asexually by binary fission, a process that gives rise to progeny that are virtually identical genetically (see Concept 7.2). That is, the offspring of cell reproduction in prokaryotes constitute a clone.
How, then, do prokaryotes evolve? Mutations occur in prokaryotes just as they do in eukaryotes, and the resulting new alleles increase genetic diversity. You might expect, therefore, that there is no way for individuals of these organisms to exchange genes as happens in sexual reproduction. It turns out, however, that prokaryotes do have a way of transferring genes between cells. This transfer of genes from one individual organism to another without sexual reproduction is called horizontal or lateral gene transfer to distinguish it from vertical gene transfer (gene transfer from parent to offspring). Along with mutation, this process generates genetic diversity among prokaryotes.
LINK
The evolutionary consequences of lateral gene transfer and its role in identifying and classifying bacterial species are discussed in Concepts 15.6 and 19.2
Bacteria exchange genes by conjugation
To illustrate genetic exchange in bacteria, let’s consider two strains of the bacterium E. coli with different alleles for each of six genes (which code for enzymes in a biochemical pathway that synthesizes a certain small molecule). The two strains have the following genotypes (remember that bacteria are haploid):
ABCdef and abcDEF
where capital letters stand for wild-type alleles that encode functional gene products and lowercase letters stand for mutant alleles that encode defective gene products. Neither strain is able to synthesize the small molecule because neither has a full complement of wild-type genes.
When the two strains are grown together in the laboratory, most of the cells produce clones. That is, almost all of the cells that grow have either genotype ABCdef or genotype abcDEF. However, out of millions of bacteria, a few occur that have the genotype ABCDEF.
Unlike the original strains, cells of genotype ABCDEF are able to synthesize the small molecule. How could these completely wild-type bacteria arise? One possibility is mutation: in the abcDEF bacteria, the a allele could have mutated to A, the b allele to B, and the c allele to C. The problem with this explanation is that a mutation at any particular point in an organism’s DNA sequence is a very rare event (about 1 in a million). The probability of all three events occurring in the same cell is extremely low—much lower than the observed rate of appearance of cells with genotype ABCDEF. So the mutant cells must have acquired wild-type genes some other way—and this turns out to be the transfer of DNA between cells.
168
Electron microscopy shows that gene transfers between bacteria can happen via physical contact between the cells (FIGURE 8.19A). Contact is initiated by a thin projection called a sex pilus (plural pili) that extends from one cell (the donor), attaches to another cell (the recipient), and draws the two cells together. Genetic material can then pass from the donor to the recipient through a thin cytoplasmic bridge called a conjugation tube. There is no reciprocal transfer of DNA from the recipient to the donor. This process is referred to as bacterial conjugation.
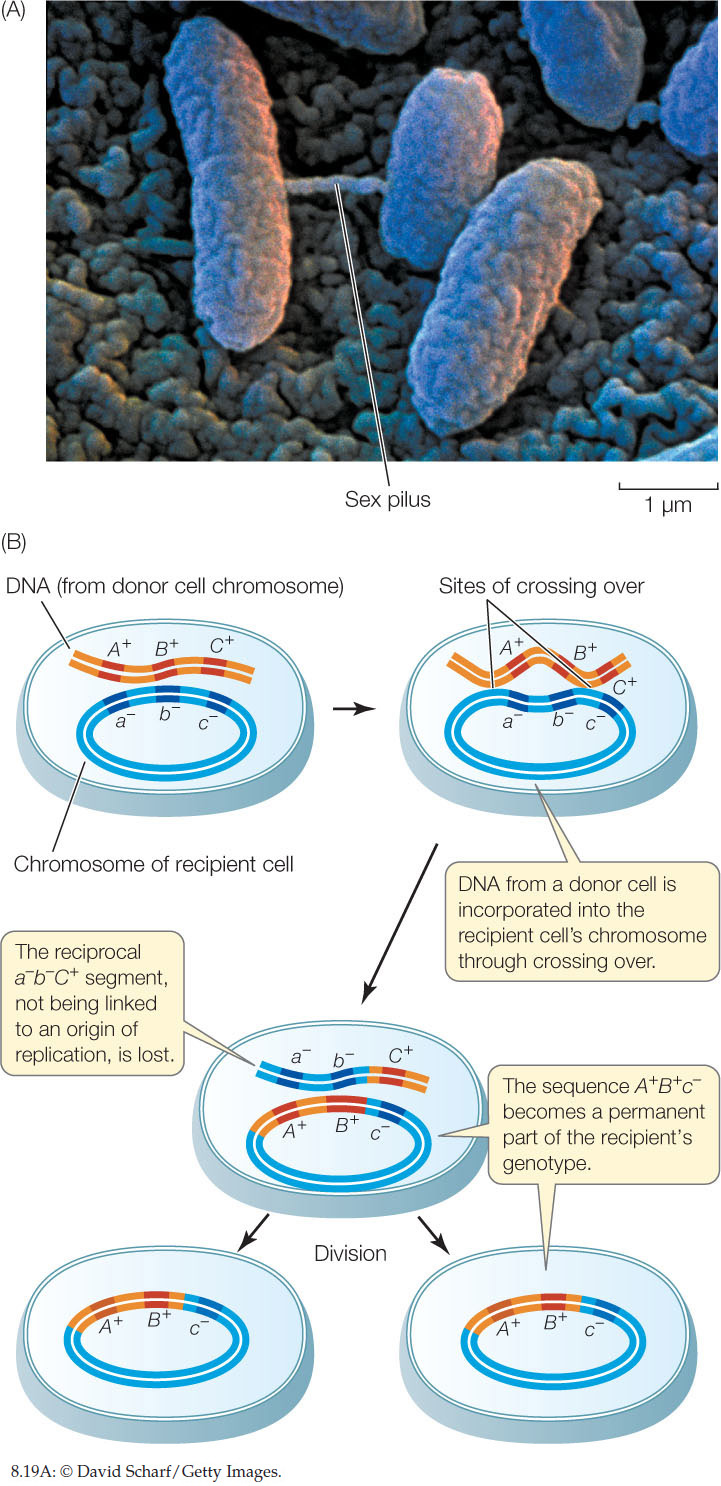
Once the donor DNA is inside the recipient cell, it can recombine with the recipient cell’s genome. In much the same way that chromosomes pair up in prophase I of meiosis, the donor DNA can line up beside its homologous genes in the recipient, and crossing over can occur. Gene(s) from the donor can become integrated into the genome of the recipient, thus changing the recipient’s genetic constitution (FIGURE 8.19B). In general, about half the transferred genes become integrated in this way. When the recipient cells proliferate, the integrated donor genes are passed on to all progeny cells, and the other transferred genes are lost. If the new combination of alleles is advantageous, the progeny of the recipient cell may be able to proliferate faster than the original strains.
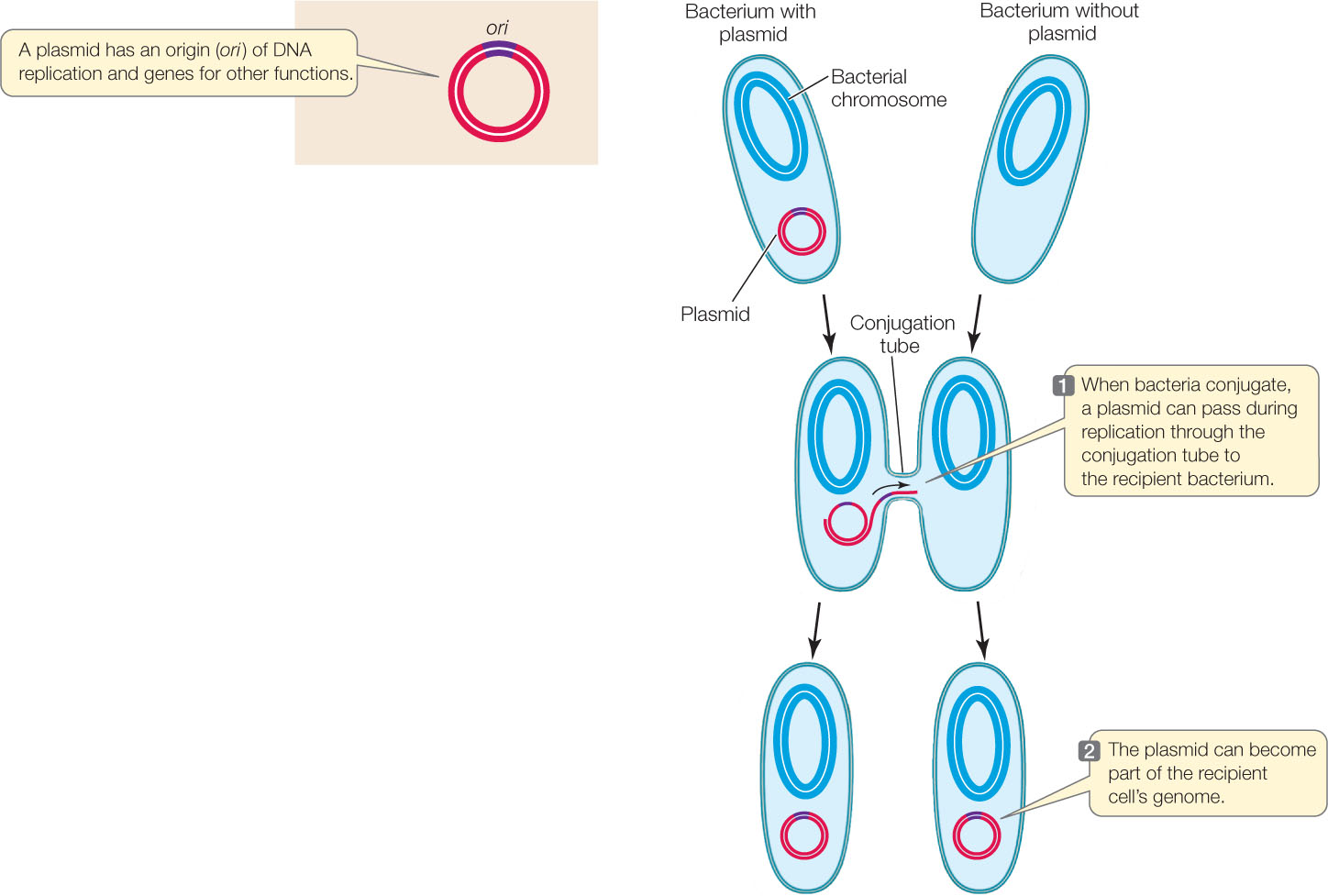
Plasmids transfer genes between bacteria
In addition to their main chromosome, many bacteria harbor additional smaller, circular DNA molecules called plasmids that replicate independently inside the cell. Plasmids typically contain at most a few dozen genes, which may fall into one of several categories, including:
- Genes for unusual metabolic capacities, such as the ability to break down hydrocarbons. Bacteria carrying these plasmids can be used to clean up oil spills.
- Genes for antibiotic resistance. Plasmids carrying such genes are called R factors, and since they can be transferred between bacteria via conjugation, they are a major threat to human health.
Plasmids can move between cells during conjugation, thereby transferring new genes to the recipient bacterium (FIGURE 8.20). A single strand of the donor plasmid is transferred to the recipient; synthesis of complementary DNA strands results in two complete copies of the plasmid, one in the donor and one in the recipient. Because plasmids can replicate independently of the main chromosome, they do not need to recombine with the main chromosome to add their genes to the recipient cell’s genome.
The evolution of drug-resistant bacteria is a major public health problem
Until the twentieth century, bacterial infections were a major scourge of humanity. With the discovery of antibiotics (particularly penicillin, which prevents the assembly of the bacterial call wall), many lethal infections were kept at bay. But over time some bacteria acquired mutations that rendered them resistant to penicillin. These bacteria had a selective advantage when faced with penicillin, and as penicillin use increased, the resistant bacteria became widespread. So scientists designed chemical variants, such as methicillin and vancomycin, to attack penicillin-resistant bacteria. With the development of strains of bacteria resistant to these antibiotics as well, the “arms race” has continued. The latest weapon against resistant bacteria is a group of antibiotics called carbapenems (for example, colistin), but bacteria resistant to these antibiotics are beginning to appear. Some resistance genes are carried on plasmids and are transferred between bacterial species by conjugation. This poses a major public health problem worldwide, since the new antibiotics are currently the last line of defense against lethal infections.
169
CHECKpoint CONCEPT 8.4
- How does recombination occur in prokaryotes?
- What is the evolutionary advantage of recombination in prokaryotes?
- What are the differences between recombination after conjugation in prokaryotes and recombination during meiosis in eukaryotes?
Question 8.2
How is hemophilia inherited, and why is it more frequent in males?
ANSWER The ancient rabbis in the opening story were dealing with male babies that bled to death when they were cut. We know this as the blood-clotting disease hemophilia. The mutant allele of the gene coding for a blood clotting factor missing in hemophilia must be recessive, because the babies’ parents did not suffer from the disease (Concept 8.1). The rabbis noted that the disease occurred in boys, and any relatives with the disease were males on the mother’s side of the family. This is because the gene for the clotting factor is carried on the human X chromosome and its inheritance is sex-linked. Females have two X chromosomes, so even if they receive a mutant X chromosome from their mother, the second X chromosome, inherited from their father, will usually provide sufficient functional clotting factor. Males, however, have a single X chromosome, always inherited from their mother (Concept 8.3). If they receive their mother’s recessive mutant chromosome (a 50–50 probability), they cannot produce clotting factor and thus suffer from hemophilia.
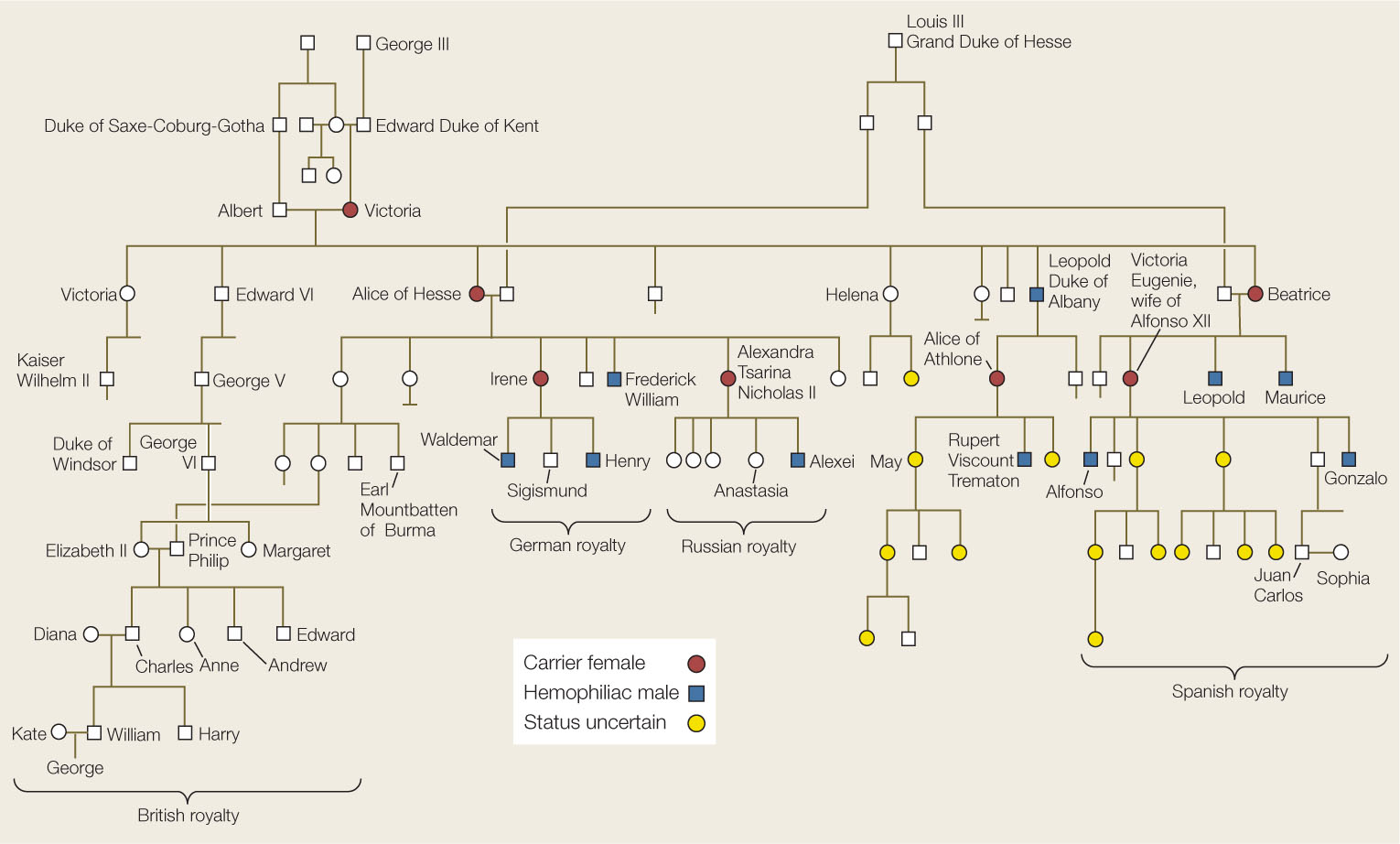
Hemophilia played a role in the history of modern Europe. England’s Queen Victoria, who ruled for much of the nineteenth century, had nine children, one of whom, Leopold, had hemophilia and died after a minor accident at age 31. The queen did not have the disease, nor was it present in any of her forbears, so it is probable that a mutation arose spontaneously on one of Victoria’s X chromosomes or that of her father (Concept 8.2). Three of Victoria’s grandchildren had hemophilia, indicating that their mothers were carriers of the mutant allele. The mutation was thus introduced into the royal families of Spain, Germany, and Russia (FIGURE 8.21). While there are many descendants of Queen Victoria still living (including Queen Elizabeth II), none have hemophilia. It remains possible, however, that some of Victoria’s female descendants are carriers.
170
171