Concept 11.2: Eukaryotic Genes Are Regulated by Transcription Factors
As we mentioned in Concept 11.1, gene expression can be regulated at several different points in the process of transcribing a gene and translating the mRNA into a protein (see Figure 11.1). In this concept we will describe the mechanisms that result in the selective transcription of specific eukaryotic genes. As in prokaryotes, eukaryotic cells must precisely regulate the expression of their genes. Some genes are constitutive (expressed in most tissues most of the time), whereas others are inducible and expressed only when needed. This is especially important in multicellular organisms with specialized cells and tissues. For example, virtually all of our cells carry the genes encoding keratin (the protein in our hair and nails) and hemoglobin. Yet keratin is made only by epithelial cells such as skin cells, and hemoglobin is made only by developing red blood cells. In contrast, all human cells express the genes that encode enzymes needed for basic metabolic activities (such as glycolysis), and all cells must synthesize certain structural proteins such as actin (a component of the cytoskeleton).
The mechanisms for regulating transcription in eukaryotes are similar conceptually to those of prokaryotes. Both types of cells use DNA–protein interactions to bring about negative and positive control of gene expression. However, there are significant differences, which generally reflect the greater complexity of eukaryotic organisms (TABLE 11.1).
Transcription factors act at eukaryotic promoters
As in bacteria, a eukaryotic promoter is a region of DNA near the 5′ end of a gene where RNA polymerase binds and initiates transcription. Eukaryotic promoters are extremely diverse and difficult to characterize, but they each contain a core promoter sequence to which the RNA polymerase binds. The most common of these is the TATA box—so called because it is rich in A-T base pairs.
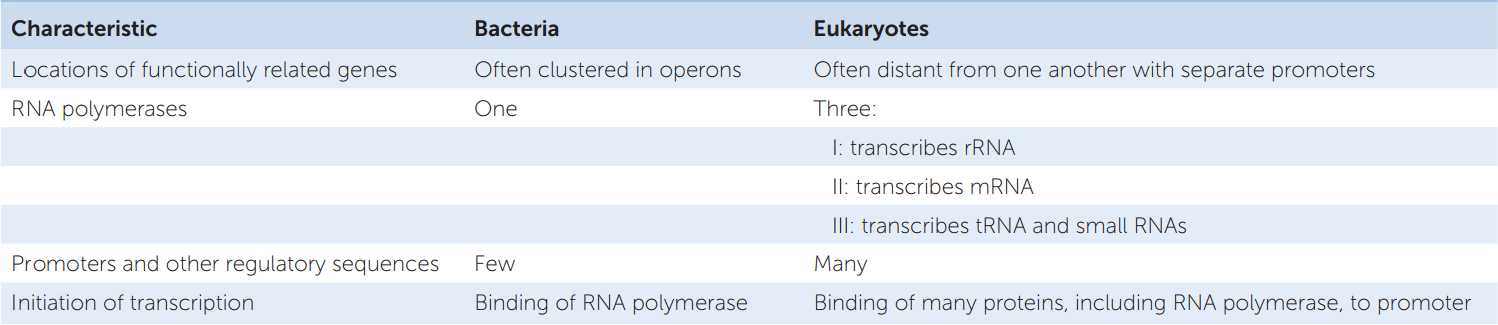
RNA polymerase II is the polymerase that transcribes the protein-coding genes in eukaryotes. It cannot bind to the promoter and initiate transcription by itself. Rather, it does so only after various general transcription factors have bound to the core promoter. General transcription factors bind to most promoters and are distinct from transcription factors that have specific regulatory effects only at certain promoters or classes of promoters. FIGURE 11.8 illustrates the assembly of the resulting transcription complex at a promoter containing a TATA box. First, the protein TFIID (“TF” stands for transcription factor) binds to the TATA box. Binding of TFIID changes both its own shape and that of the DNA, presenting a new surface that attracts the binding of other transcription factors. RNA polymerase II binds only after several other proteins have bound to the complex.

Go to ANIMATED TUTORIAL 11.3 Initiation of Transcription
PoL2e.com/at11.3
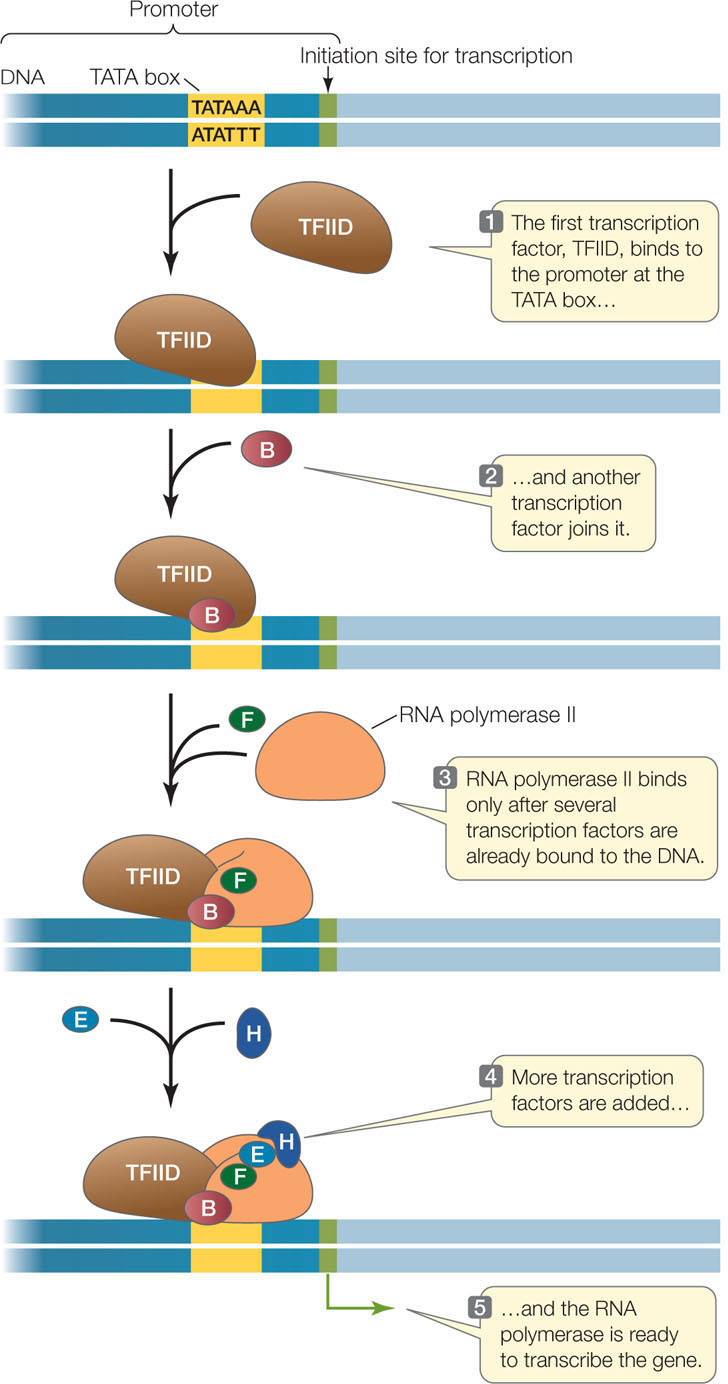
The core promoter sequence is bound by general transcription factors that are needed for the expression of all RNA polymerase II-transcribed genes. Other sequences that are (usually) found in or near promoter regions are specific to only a few genes and are recognized by specific transcription factors. These transcription factors may be positive regulators (activators) or negative regulators (repressors) of transcription:
223
Such transcription factors may be present only in certain cell types, or they may be present in all cells but activated by specific signals. DNA sequences that bind activators are called enhancers, and those that bind repressors are called silencers. Some enhancers and silencers occur near the core promoter, and others can be as far as 20,000 base pairs away. When the activators or repressors bind to these DNA sequences, they interact with the RNA polymerase complex, causing the DNA to bend. Often many such binding proteins are involved, and the combination of factors present determines whether transcription is initiated. With about 2,000 different transcription factors in humans, there are many possibilities for regulation.
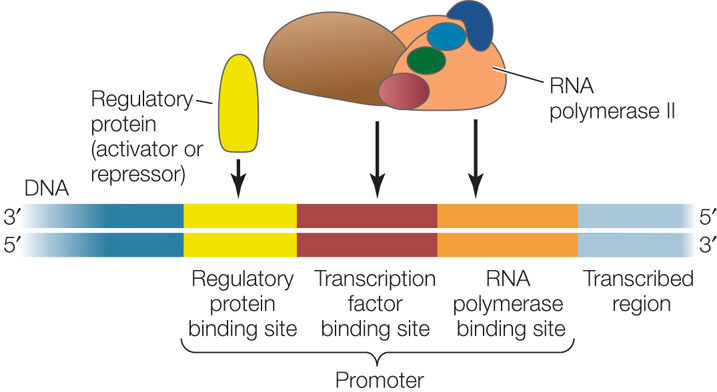
How do transcription factors recognize a specific nucleotide sequence in DNA? To answer this question, let’s look at a specific example. NFATs (nuclear factors of activated T cells) are a group of transcription factors that control the expression of genes essential for the immune response (see Chapter 39). NFAT proteins bind to a 12-bp recognition sequence near the promoters of these genes, with the sequence CGAGGAAAATTG (FIGURE 11.9). Recall that there are atoms in the bases of DNA that are available for hydrogen bonding but are not involved in base pairing (see Figure 9.6). These atoms are important in the interactions between an NFAT and the DNA. In addition, there are hydrophobic interactions between the rings in the DNA bases and some amino acid R groups in the protein. As for an enzyme and its substrate (see Concept 3.3), there is an induced fit between the NFAT and the DNA, such that the protein undergoes a conformational change after binding begins.
The base sequence of a binding site on DNA determines the arrangement of chemical groups available for hydrogen bonding and hydrophobic interactions with DNA-binding proteins; this is the basis of the specificity of DNA–protein interactions.
The expression of transcription factors underlies cell differentiation
During the development of a complex organism from fertilized egg to adult, cells become more and more differentiated (specialized). Differentiation is brought about in many cases by changes in gene expression, resulting from the activation (and inactivation) of transcription factors. We will discuss this topic in more detail in Chapter 14. For now, remember that virtually all differentiated cells contain the entire genome, and that their specific characteristics arise from differential gene expression.
Currently there is great interest in cellular therapy: providing new, functional cells to patients who have diseases that involve the degeneration of certain cell types. An example is Alzheimer’s disease, which involves the degeneration of neurons in the brain. Because of the possibility of immune system rejection (see Chapter 39), it would be optimal if patients could receive their own cells, modified in some way to be functional. Since specialized functions are under the control of transcription factors, turning readily available cells into a particular desired cell type might be achieved by altering transcription factor expression. Marius Wernig and his colleagues at Stanford University have made important progress toward this goal (FIGURE 11.10). They took skin fibroblasts from mice and manipulated the expression of transcription factors in the cells to change them into neurons. By repeating their experiments on human fibroblasts, they have brought cellular therapy closer to reality.
224
Investigation
HYPOTHESIS
Expression of neuron-specific transcription factors in fibroblasts will turn the latter into neurons.
METHOD
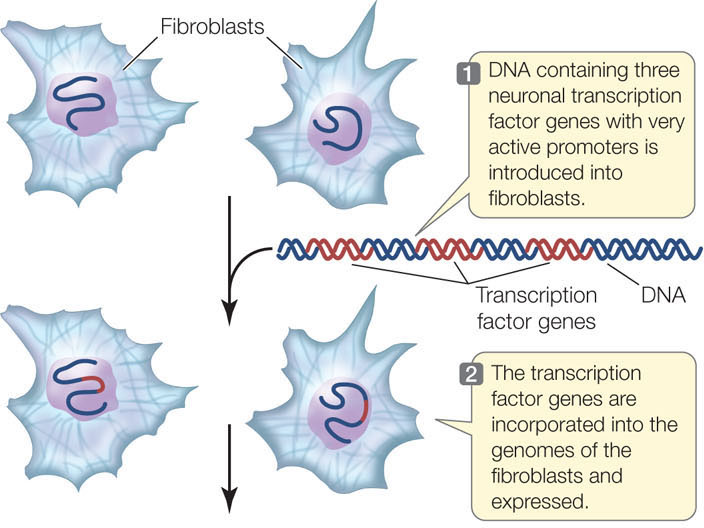
RESULTS
After 6 days, the fibroblasts develop into functional neurons, which form characteristic synapses with one another.
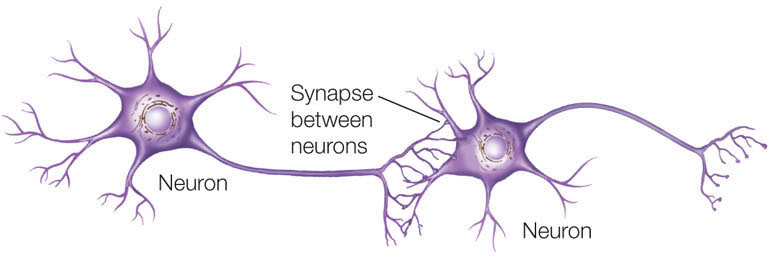
CONCLUSION
The expression of just three transcription factors is sufficient to transform a fibroblast into a neuron.
ANALYZE THE DATA
Fibroblasts are active in cell division; neurons are not. In addition to morphology, the lack of cell division was used as a criterion to show that the transformed cells were neurons. The rate of cell division in the transformed cells was measured by the incorporation of the labeled nucleotide bromo-deoxyuridine (BrdU) into DNA. The percentage of labeled (hence dividing) cells is shown in the graph.
- Was cell division stopped in the transformed cells? Explain your answer.
- The error bars are standard deviations. What statistical test would you use to show whether the difference between the two cell populations was significant? See Appendix B for a Statistics Primer.
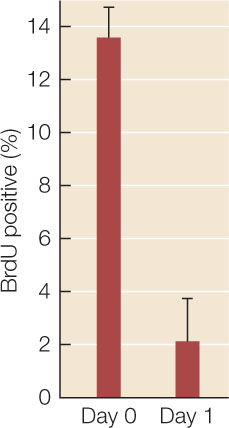
Go to LaunchPad for discussion and relevant links for all INVESTIGATION figures.
aT. Vierbuchen et al. 2010. Nature 463: 1035–1041.
LINK
The basis for rejection of nonself cells is described in Concept 39.5. Additional approaches used in cellular therapy are discussed in Concept 14.1
Transcription factors can coordinate the expression of sets of genes
We have seen that prokaryotes can coordinate the regulation of several genes by arranging them in an operon. In addition, bacteria can coordinate the expression of groups of genes using sigma factors, which guide RNA polymerase to particular classes of promoters. This latter mechanism is also used in eukaryotes to coordinately regulate genes that may be far apart, even on different chromosomes. The expression of genes can be coordinated if they share regulatory sequences that bind the same transcription factors.
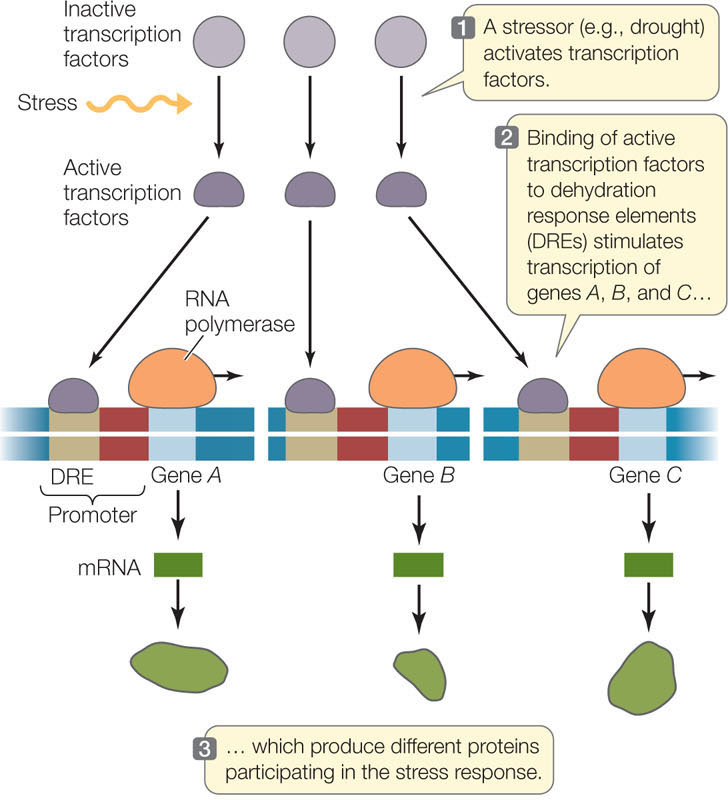
This type of coordination is used by organisms to respond to stress—for example, by plants in response to drought. Under conditions of drought stress, a plant must simultaneously synthesize numerous proteins whose genes are scattered throughout the genome. The synthesis of these proteins comprises the stress response. To coordinate expression, each of these genes has a specific regulatory sequence near its promoter called the dehydration response element (DRE). In response to drought, a transcription factor changes so that it binds to this element and stimulates mRNA synthesis (FIGURE 11.11). The dehydration response proteins not only help the plant conserve water but also protect the plant against freezing or excess salt in the soil. This finding has considerable importance for agriculture because crops are often grown under less than optimal conditions.
225
LINK
Dehydration response proteins are one of many adaptations to drought stress found among the plants; see Concept 28.3
Eukaryotic viruses can have complex life cycles
Eukaryotes are susceptible to infections by various kinds of viruses that have a variety of life cycle strategies. These viral life cycles can be very efficient—for example, the poliovirus completes its life cycle (from infection to release of new particles) in 4–6 hours, and each dying host cell can release up to 10,000 new particles. Compare this with the 24-hour cell cycle typical of dividing human cells.
The viruses that infect eukaryotic cells may have genomes of single- or double-stranded DNA or RNA. Some viral life cycles can be quite complex. As an example, we focus here on human immunodeficiency virus (HIV), the infective agent that causes acquired immunodeficiency syndrome (AIDS) in humans. HIV typically infects only cells of the immune system that express a surface receptor called CD4. The virion is enclosed within a phospholipid membrane derived from its previous host cell. Proteins in the membrane are involved in the infection of new host cells, which HIV enters by direct fusion of the viral envelope with the host’s cell membrane (FIGURE 11.12).
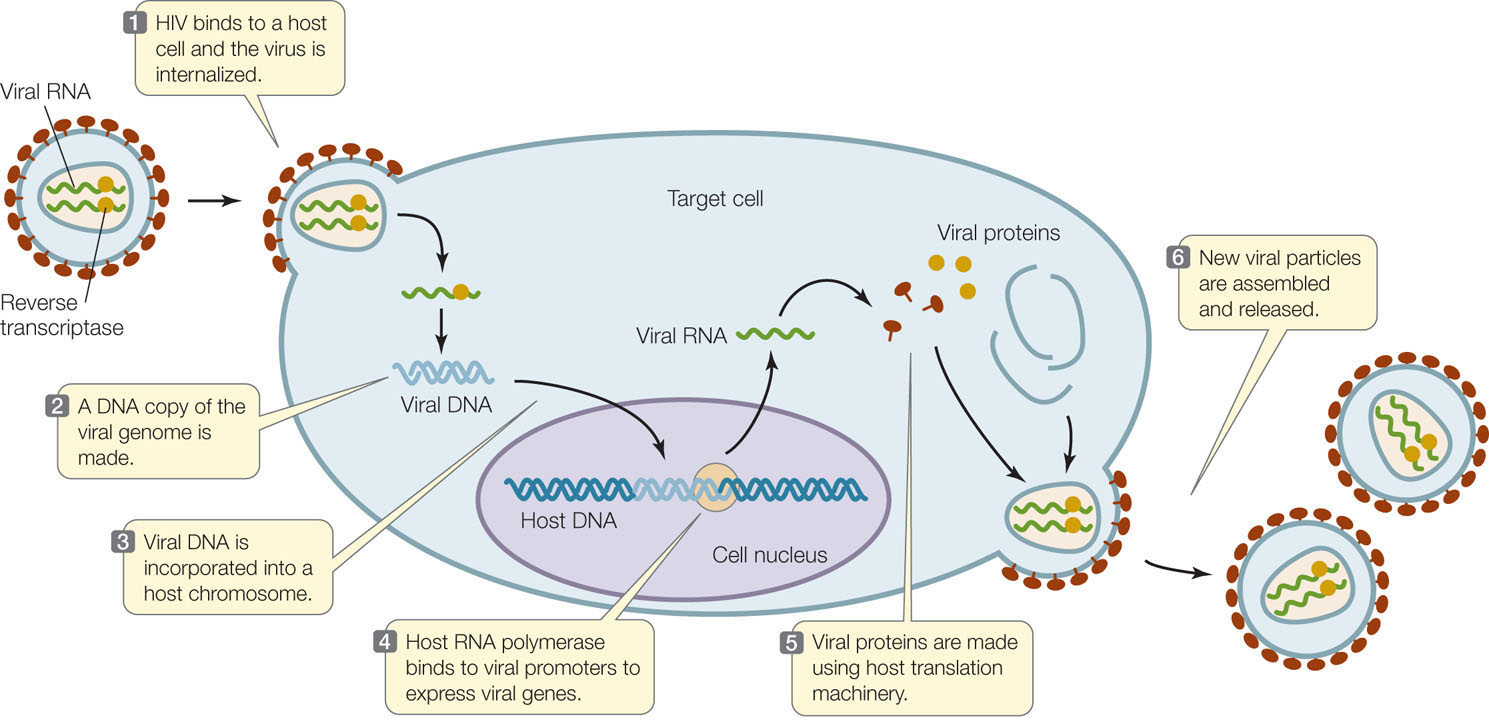
HIV is a retrovirus: its genome is single-stranded RNA, and it carries within the virion an enzyme called reverse transcriptase. Shortly after infection, the reverse transcriptase makes a DNA strand that is complementary to the RNA, while at the same time degrading the RNA and making a second DNA strand that is complementary to the first. The resulting double-stranded DNA becomes integrated into the host’s chromosome. The integrated viral DNA is called a provirus.
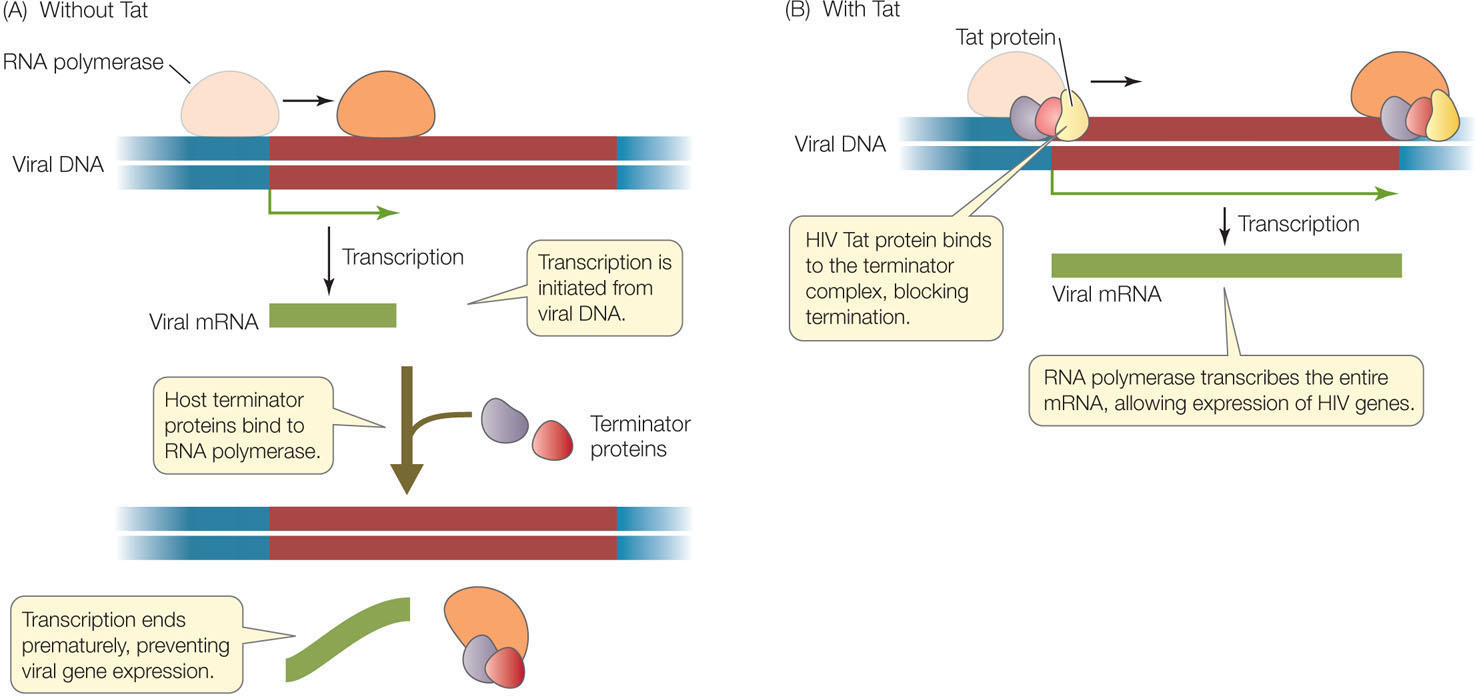
The provirus resides permanently in the host chromosome and can remain in an inactive state for years. During this time transcription of the viral DNA is initiated, but host cell proteins called termination factors prevent the RNA from elongating, and transcription is terminated prematurely (FIGURE 11.13A). Under some circumstances, the level of transcription initiation increases and some viral RNA is made. One of the viral genes encodes a protein called Tat (Transactivator of transcription), which binds to the 5′ end of the viral RNA. As a result of Tat binding, the production of full-length viral RNA is dramatically increased (FIGURE 11.13B), and the rest of the viral reproductive cycle is able to proceed. It was only after the discovery of this mechanism in HIV and similar viruses that researchers found that many eukaryotic genes are regulated at the level of transcription elongation.
226
CHECKpoint CONCEPT 11.2
- How do transcription factors regulate gene expression? How do the roles of transcription factors compare with the roles of proteins that regulate prokaryotic operons?
- What would be the effect of the inhibition of reverse transcriptase on infection of a cell with HIV?
We have discussed some of the mechanisms that cells and viruses use to control gene transcription. These mechanisms involve the interaction of regulatory proteins with specific DNA sequences. However, eukaryotes have other mechanisms for controlling gene expression that do not depend on specific DNA sequences. We will discuss these mechanisms in the next concept.