Concept 19.4: Viruses Have Evolved Many Times
Some biologists do not think of viruses as living organisms, primarily because they are not cellular and must depend on cellular organisms for basic life functions such as replication and metabolism. But viruses are derived from the cells of living organisms. They use the same essential forms of genetic information storage and transmission as do cellular organisms. Viruses infect all cellular forms of life—bacteria, archaea, and eukaryotes. They replicate, mutate, evolve, and interact with other organisms, often causing serious diseases in their hosts. Finally, viruses clearly evolve independently of other organisms, so it is almost impossible not to treat them as a part of life.
Viruses are abundant in many environments. In some freshwater and marine ecosystems, they can occur at densities of up to 10 million viruses per milliliter of water. Biologists estimate that there are approximately 1031 individual virus particles on Earth—about 1,000 times the number of cellular organisms on the planet. Viruses have an enormous effect on the ecology of the oceans. Every day, about one-half of the bacteria in the oceans are killed by viruses. Huge marine blooms of bacteria, such as the Vibrio bloom that produced the milky seas described at the opening of this chapter, do not last for long because viral blooms soon follow the initial bacterial bloom. As the viruses increase, they begin to kill bacteria faster than the bacteria can reproduce.
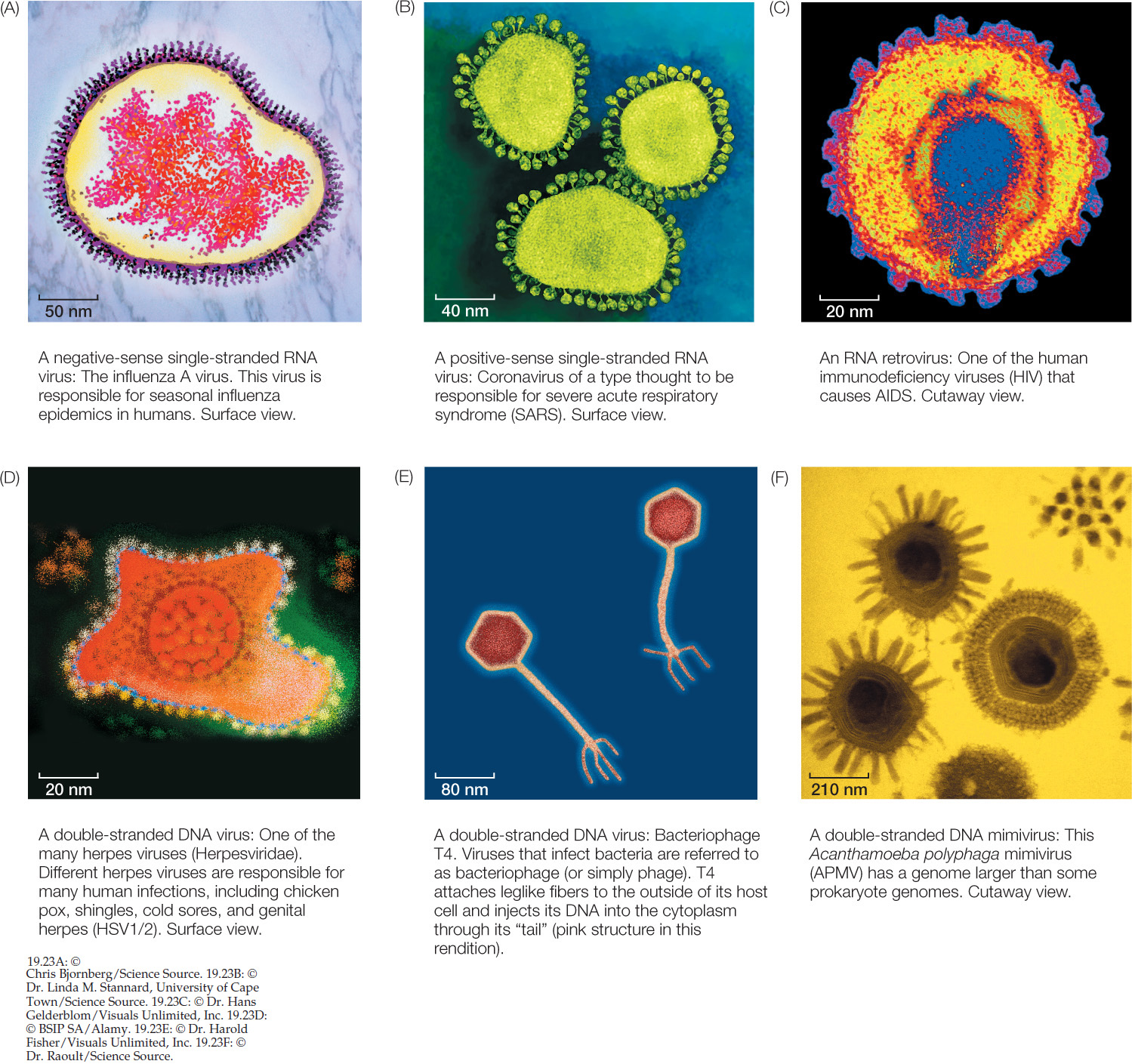
Although viruses are everywhere and play an important role in many ecosystems, many aspects of their ecology and evolution are still poorly known. For example, several factors make virus phylogeny difficult to resolve. The tiny size of many virus genomes restricts the phylogenetic analyses that can be conducted to relate viruses to cellular organisms. Even viruses with large genomes often contain many genes that are difficult to align with the genomes of cellular organisms. Their rapid mutation rate, which results in rapid evolution of virus genomes, tends to cloud evolutionary relationships over long periods. There are no known fossil viruses (viruses are too small and delicate to fossilize), so the paleontological record offers no clues to virus origins. Finally, viruses are highly diverse (FIGURE 19.23). Several lines of evidence support the hypothesis that viruses have evolved repeatedly within each of the major groups of life. The difficulty in resolving deep evolutionary relationships of viruses makes a phylogeny-based classification difficult. Instead, viruses are placed in one of several functionally similar groups on the basis of the structure of their genomes (for example, whether the genomes are composed of RNA or DNA, and are double- or single-stranded). Most of these defined groups are not thought to represent monophyletic taxa, however.
Many RNA viruses probably represent escaped genomic components
Although viruses are now obligate parasites of cellular species, many viruses may once have been cellular components involved in basic cellular functions—that is, they may be “escaped” components of cellular life that now evolve independently of their hosts.
Negative-Sense Single-Stranded RNA Viruses
A case in point is a class of viruses whose genome is composed of single-stranded negative-sense RNA: RNA that is the complement of the mRNA needed for protein translation. Many of these negative-sense single-stranded RNA viruses have only a few genes, including one for an RNA-dependent RNA polymerase that allows them to make mRNA from their negative-sense RNA genome. Modern cellular organisms cannot generate mRNA in this manner (at least in the absence of viral infections), but scientists speculate that single-stranded RNA genomes may have been common in the distant past, before DNA became the primary molecule for genetic information storage.
A self-replicating RNA polymerase gene that began to replicate independently of a cellular genome could conceivably acquire a few additional protein-coding genes through recombination with its host’s DNA. If one or more of these genes were to foster the development of a protein coat, the virus might then survive outside the host and infect new hosts. It is believed that this scenario has been repeated many times independently across the tree of life, given that many of the negative-sense single-stranded RNA viruses that infect organisms from bacteria to humans are not closely related to one another. In other words, negative-sense single-stranded RNA viruses do not represent a distinct taxonomic group, but rather exemplify a particular process of cellular escape that probably happened many different times.
397
Familiar examples of negative-sense single-stranded RNA viruses include the viruses that cause measles, mumps, rabies, and influenza (see Figure 19.23A).
Positive-Sense Single-Stranded RNA Viruses
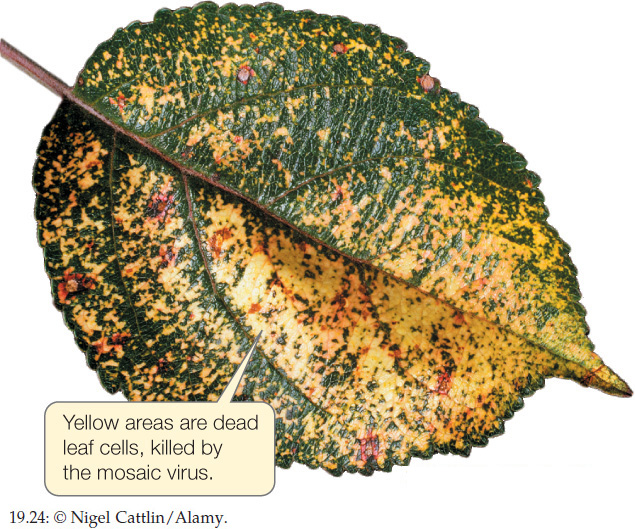
The genome of another type of single-stranded RNA virus is composed of positive-sense RNA. Positive-sense genomes are already set for translation; no replication of the genome to form a complement strand is needed before protein translation can take place. Positive-sense single-stranded RNA viruses (see Figure 19.23B) are the most abundant and diverse class of viruses. Most of the viruses that cause diseases in crop plants are members of this group. These viruses kill patches of cells in the leaves or stems of plants, leaving live cells amid a patchwork of discolored dead tissue (giving them the name of mosaic or mottle viruses; FIGURE 19.24). Other viruses in this group infect bacteria, fungi, and animals. Human diseases caused by positive-sense single-stranded RNA viruses include polio, hepatitis C, and the common cold. As is true of the other functionally defined groups of viruses, these viruses appear to have evolved multiple times across the tree of life from different groups of cellular ancestors.
398
APPLY THE CONCEPT: Viruses have evolved many times
When Gram staining revealed the first mimivirus (discovered living inside an amoeba), it was mistakenly identified as a parasitic Gram-positive bacterium. It was soon discovered that this species was not cellular, however, and it was reclassified as a virus. The graph below shows the genome size and number of protein-coding genes for various species of bacteria. Use the graph to answer the following questions.
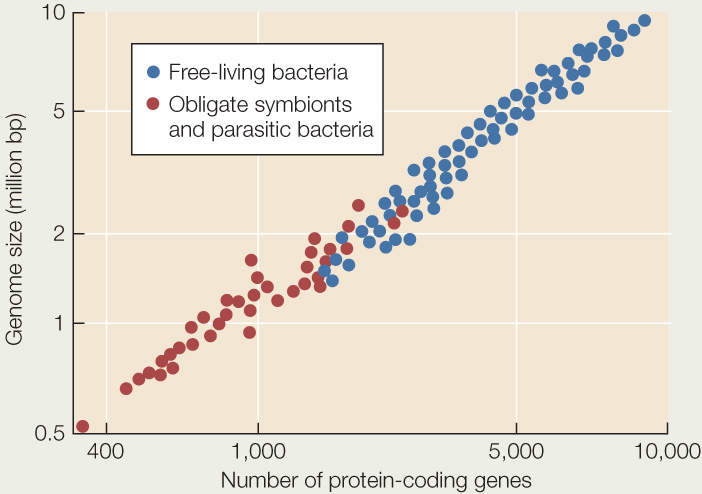
- What is a likely explanation for the generally smaller genome size and smaller number of protein-coding genes in parasitic bacteria than in most free-living species? Can you form a hypothesis about which species of parasitic bacteria are likely to have existed for the longest time as parasites?
- The genome of Acanthamoeba polyphaga mimivirus (the first discovered mimivirus) is 1,181,404 base pairs, encompassing 911 protein-coding genes. Plot the relevant data for this mimivirus on the graph. How does your result fit with the hypothesis that the mimivirus evolved from a parasitic bacterium?
- Earlier in this chapter we described a minute, parasitic group of archaea, the Nanoarchaeota (see Figure 19.18). The genome of one species, Nanoarchaeum equitans, is 490,885 base pairs and contains 536 protein-coding genes. Plot the relevant data for this species on the graph. How does the genome of this parasitic archaea species differ from the genomes of the parasitic bacteria? From the genome of the mimivirus?
RNA Retroviruses
The RNA retroviruses are best known as the group that includes the human immunodeficiency viruses (HIV; see Figure 19.23C). Like the previous two categories of viruses, RNA retroviruses have genomes composed of single-stranded RNA and probably evolved as escaped cellular components.
Retroviruses are so named because they regenerate themselves by reverse transcription. When the retrovirus enters the nucleus of its vertebrate host, viral reverse transcriptase produces complementary DNA (cDNA) from the viral RNA genome and then replicates the single-stranded cDNA to produce double-stranded DNA. Another virally encoded enzyme called integrase catalyzes the integration of the new piece of double-stranded viral DNA into the host’s genome. The viral genome is then replicated along with the host cell’s DNA. The integrated retroviral DNA is known as a provirus.
Retroviruses are only known to infect vertebrates, although genomic elements that resemble portions of these viruses are a component of the genomes of a wide variety of organisms, including bacteria, plants, and many animals. Several retroviruses are associated with the development of various forms of cancer, as cells infected with these viruses are more likely to undergo uncontrolled replication.
As retroviruses become incorporated into the genomes of their hosts, many become nonfunctional copies that are no longer expressed as functional viruses. These sequences may provide a record of ancient viral infections that plagued our ancestors. Humans, for example, carry about 100,000 fragments of endogenous retroviruses in our genome. These fragments make up about 8 percent of our DNA—a considerably larger fraction of our genome than the fraction that comprises all our protein-coding genes (about 1.2 percent of our genome).
Double-Stranded RNA Viruses
Double-stranded RNA viruses may have evolved repeatedly from single-stranded RNA ancestors—or perhaps vice versa. These viruses, which are not closely related to one another, infect organisms from throughout the tree of life. Many plant diseases are caused by double-stranded RNA viruses. Other viruses of this type cause many cases of infant diarrhea in humans.
399
Some DNA viruses may have evolved from reduced cellular organisms
Another class of viruses is composed of viruses that have a double-stranded DNA genome (see Figure 19.23D–F). This group is also almost certainly polyphyletic (with many independent origins). Many of the common phage that infect bacteria are double-stranded DNA viruses, as are the viruses that cause smallpox and herpes in humans.
Some biologists think that at least some of the DNA viruses may represent highly reduced parasitic organisms that have lost their cellular structure as well as their ability to survive as free-living species. For example, the mimiviruses (see Figure 19.23F) have a genome in excess of a million base pairs of DNA that encode more than 900 proteins. This genome is similar in size to the genomes of many parasitic bacteria and about twice as large as the genome of the smallest bacteria. The recently discovered pandoraviruses have even larger genomes (up to about 2.5 million base pairs)—larger than the smallest genomes of eukaryotes. Phylogenetic analyses of DNA viruses suggest that they have evolved repeatedly from cellular organisms, possibly including major branches of life that are now extinct. Furthermore, recombination among different viruses may have allowed the exchange of various genetic modules, further complicating the history and origins of these viruses.
Viruses can be used to fight bacterial infections
Although some viruses cause devastating diseases, other viruses have been used to fight disease. Most bacterial diseases are treated today with antibiotics. But antibiotics were first discovered in the 1930s, and they were not widely used to treat bacterial diseases until the 1940s. So antibiotics were not yet available during World War I, when bacterial infections plagued the battlefields. Battlefield wounds were often infected by bacteria, and in the absence of antibiotics, these infections often led to the loss of limbs and lives. While trying to find a way to combat this problem, a physician named Felix d’Herelle discovered the first evidence of viruses that attack bacteria. He named these viruses bacteriophage, or “eaters of bacteria.” Herelle extracted bacteriophage from the stool of infected patients. He then used these extracts to treat patients with deadly bacterial infections, including dysentery, cholera, and bubonic plague. This practice became known as phage therapy. After the war, phage therapy was widely used among the general public to treat bacterial infections of the skin and intestines.

Go to MEDIA CLIP 19.3 Bacteriophages Attack E. coli
PoL2e.com/mc19.3
Phage therapy was mostly replaced by the use of antibiotics in the 1930s and 1940s as physicians grew concerned about treating patients with live viruses. Phage therapy continued to be used in the Soviet Union but largely disappeared from Western medical practice. Today, however, many antibiotics are losing their effectiveness as bacterial pathogens evolve resistance to these drugs. Phage therapy is once again an active area of research, and it is likely that bacteriophage will become increasingly important as weapons against bacterial diseases. One advantage that bacteriophage may have over antibiotics is that, like bacteria, bacteriophage can evolve. As bacteria evolve resistance to a strain of bacteriophage, biologists can select for new strains of bacteriophage that retain their effectiveness against the pathogens. In this way, biologists are using their understanding of evolution to combat the problem of antibiotic-resistant bacteria.
CHECKpoint CONCEPT 19.4
- Why is it difficult to place viruses precisely within the tree of life?
- What are the two main hypotheses of virus origins?
- How can viruses be used to treat some human diseases?
It appears that the enormous diversity of viruses is, at least in part, a result of their multiple origins from many different cellular organisms. It may be best to view viruses as spinoffs from the various branches on the tree of life—sometimes evolving independently of cellular genomes, sometimes recombining with them. One way to think of viruses is as the “bark” on the tree of life: certainly an important component all across the tree, but not quite like the main branches.
Question 19.2
What adaptive advantage does bioluminescence provide to Vibrio bacteria?
ANSWER Although marine Vibrio can live independently, they thrive inside the guts of fish and other marine animals. Inside a fish, Vibrio cells attach themselves to food particles, including phytoplankton, and are eventually expelled into the ocean as waste. How can they get back into another fish gut? The bioluminescent glow produced by a dense colony of free-living Vibrio growing on phytoplankton attracts fish, which consume the phytoplankton and thus ingest the bacteria—which gets the bacteria into a new host fish.
400