Concept 23.3: Protostomes Have an Anterior Brain and a Ventral Nervous System
The protostomes are a highly diverse group of animals. Although their body plans are extremely varied, they are all bilaterally symmetrical animals whose bodies exhibit two major derived traits:
- An anterior brain that surrounds the entrance to the digestive tract
- A ventral nervous system consisting of paired or fused longitudinal nerve cords
Other aspects of protostome body organization differ widely from group to group. Although the common ancestor of the protostomes likely had a coelom, subsequent modifications of the coelom distinguish many protostome lineages. In at least one protostome lineage (the flatworms), the coelom has been lost (that is, the flatworms reverted to an acoelomate state). Some lineages are characterized by a pseudocoel (see Figure 23.4B). In two of the most prominent protostome groups, the coelom has been highly modified:
- The arthropods lost the ancestral condition of the coelom over the course of evolution. Their internal body cavity has become a hemocoel, or “blood chamber,” in which fluid from an open circulatory system bathes the internal organs before returning to blood vessels.
- Most mollusks have an open circulatory system with some of the attributes of the hemocoel, but they retain vestiges of an enclosed coelom around their major organs.
The evolutionary relationships of one small group of protostomes, the arrow worms (see Figure 23.1), have been debated for many years. Although recent gene sequence studies clearly identify arrow worms as protostomes, there remains some question as to their exact placement within the protostomes. The 180 living species of arrow worms are small (3 mm–12 cm) marine predators of planktonic protists and small fish.
The protostomes can be divided into two major clades—the lophotrochozoans and the ecdysozoans—largely on the basis of DNA sequence analysis. However, there are also some morphological characteristics that unite most members of these two groups.

Go to ANIMATED TUTORIAL 23.2 An Overview of the Protosomes
PoL2e.com/at23.2
Cilia-bearing lophophores and trochophore larvae evolved among the lophotrochozoans
Lophotrochozoans derive their name from two different features that involve cilia: a feeding structure known as a lophophore and a free-living larva known as a trochophore. Neither feature is universal for all lophotrochozoans, however.
Several distantly related groups of lophotrochozoans (including bryozoans, brachiopods, and phoronids) have a lophophore, a circular or U-shaped ring of ciliated, hollow tentacles around the mouth (FIGURE 23.11A). This complex structure is an organ for both food collection and gas exchange. The lophophore appears to have evolved independently several times, although it may be an ancestral feature that has been lost in many groups. Nearly all animals with a lophophore are sessile as adults, using the lophophore’s tentacles and cilia to capture small floating organisms from the water.

481

Go to MEDIA CLIP 23.3 Feeding with a Lophophore
PoL2e.com/mc23.3
Some lophotrochozoans, especially in their larval form, use cilia for locomotion. The larval form known as a trochophore moves by beating a band of cilia:
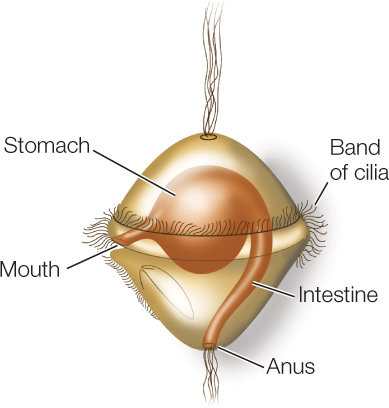
This movement of cilia also brings plankton closer to the larva, where the plankton can be captured and ingested (similar in function to the cilia of the lophophore). Trochophore larvae are found among many of the major groups of lophotrochozoans, including the mollusks, annelids, ribbon worms, and bryozoans. This larval form was probably present in the common ancestor of lophotrochozoans, although it has been subsequently lost in several lineages.
Lophotrochozoans range from relatively simple animals with a blind gut (that is, a gut with a single opening that both takes in food and expels wastes) and no internal transport system to animals with a complete gut (having separate entrance and exit openings) and a complex internal transport system. A number of these groups exhibit wormlike bodies, but the lophotrochozoans encompass a wide diversity of morphologies, including a few groups with external shells. Included among the lophotrochozoans are species-rich groups such as flatworms, annelids, and mollusks, along with many less well known groups, some of which have only recently been discovered.
Bryozoans
Most of the 5,500 species of bryozoans (“moss animals”) are colonial animals that live in a “house” made of material secreted by the external body wall. Almost all bryozoans are marine, although a few species occur in fresh or brackish water. A bryozoan colony consists of many small (1–2 mm) individuals connected by strands of tissue along which nutrients can be moved (FIGURE 23.11B). The colony is created by the asexual reproduction of its founding member, and a single colony may contain as many as 2 million individuals. Rocks in coastal regions in many parts of the world are covered with luxuriant growths of bryozoans. Some bryozoans create miniature reefs in shallow waters. In some species, the individual colony members are differentially specialized for feeding, reproduction, defense, or support.
Bryozoans can also reproduce sexually. Sperm are released into the water, which carries the sperm to other individuals. Eggs are fertilized internally; developing embryos are brooded before they exit as larvae to seek suitable sites for attachment to the substrate.
Flatworms
Most of the 30,000 species of flatworms are tapeworms and flukes; members of these two groups are internal parasites, particularly of vertebrates (FIGURE 23.12A). Because they absorb digested food from the guts of their hosts, many parasitic flatworms lack digestive tracts of their own. Some cause serious human diseases, such as schistosomiasis, which is common in parts of Asia, Africa, and South America. The species that causes this devastating disease has a complex life cycle involving both freshwater snails and mammals as hosts. Other flatworms are external parasites of fishes and other aquatic vertebrates. The turbellarians include most of the free-living species (FIGURE 23.12B).
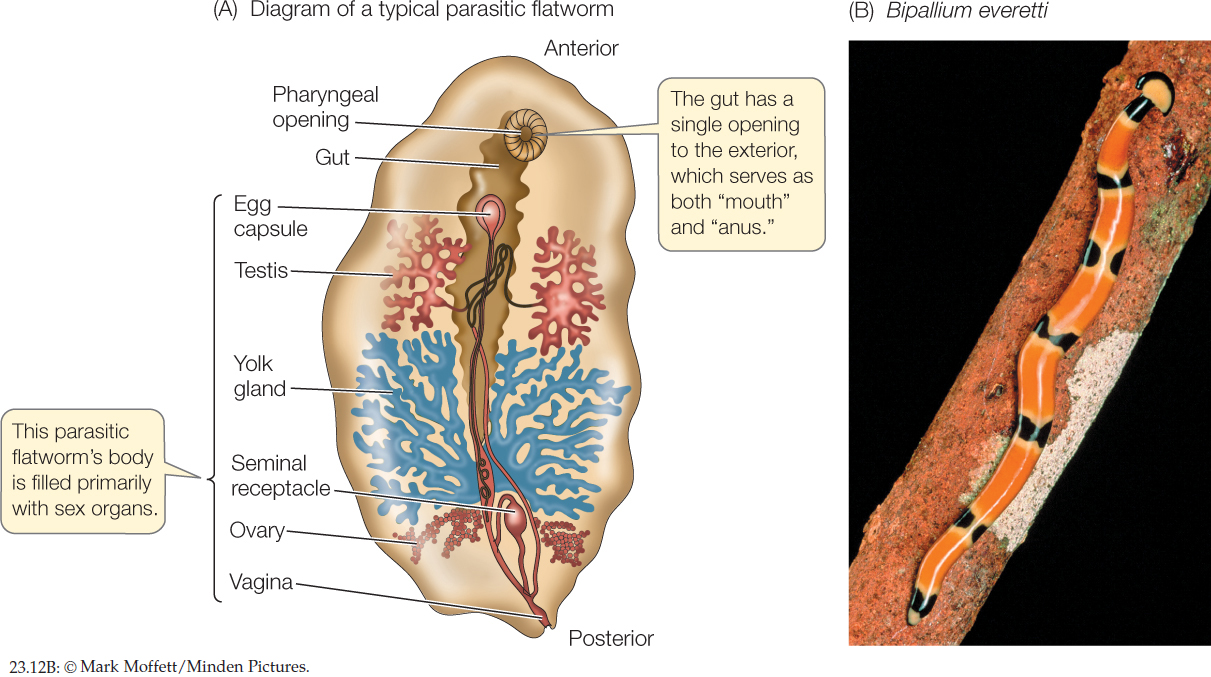
482
Flatworms lack specialized organs for transporting oxygen to their internal tissues. Lacking a gas transport system, each cell must be near a body surface, a requirement met by the dorsoventrally flattened body form. In flatworms that have a digestive tract, this consists of a mouth opening into a blind sac. The sac is often highly branched, forming intricate patterns that increase the surface area available for the absorption of nutrients. Most free-living flatworms are cephalized, with a head bearing chemoreceptor organs, two simple eyes, and a small brain composed of anterior thickenings of the longitudinal nerve cords. Free-living flatworms glide over surfaces, powered by broad bands of cilia.
Rotifers
Most species of rotifers are tiny—50–500 μm long, smaller than some ciliate protists—but they have specialized internal organs (FIGURE 23.13). A complete gut passes from an anterior mouth to a posterior anus. The body cavity is a pseudocoel that functions as a hydrostatic skeleton. Rotifers typically propel themselves through the water by means of rapidly beating cilia rather than by muscular contraction.
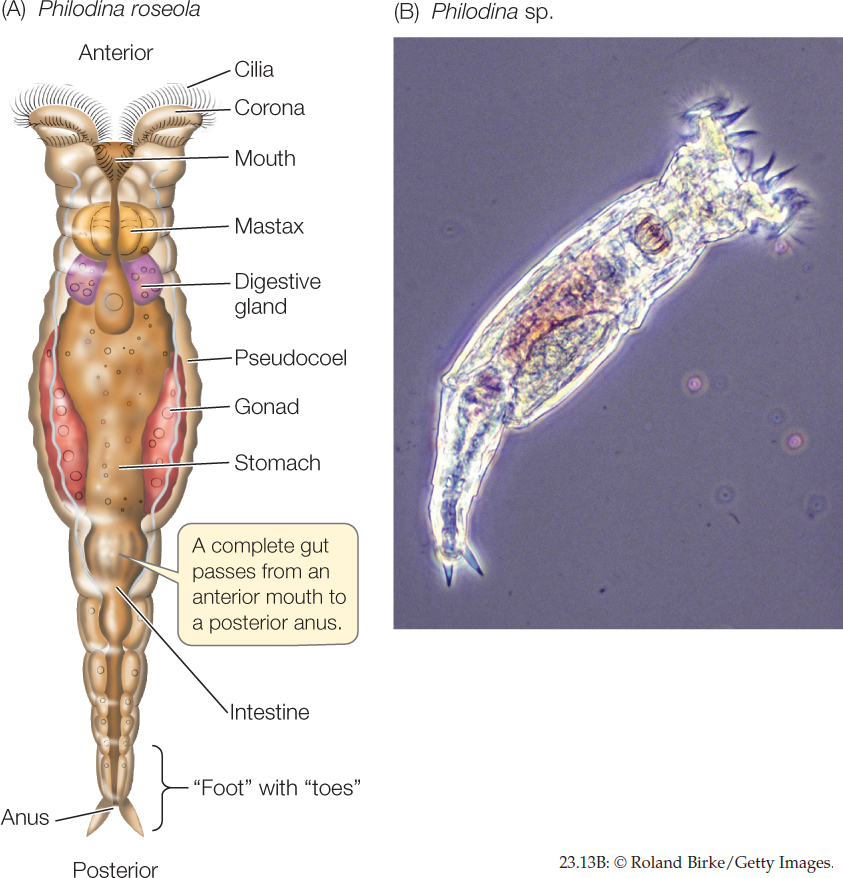
The most distinctive organ of rotifers is a conspicuous ciliated organ called the corona, which surmounts the head of many species. Coordinated beating of the cilia sweeps particles of organic matter from the water into the animal’s mouth and down to a complicated structure called the mastax, in which food is ground into small pieces. By contracting muscles around the pseudocoel, a few rotifer species that prey on protists and small animals can protrude the mastax through their mouth and seize small objects with it.
Most species of rotifers live in fresh water. Some species rest on the surfaces of mosses or lichens in a desiccated, inactive state until it rains. When rain falls, they absorb water and become mobile, feeding in the films of water that temporarily cover the plants. Most rotifers live no longer than a few weeks.
Both males and females are found in some species of rotifers, but only females are known among the bdelloid rotifers (the b in “bdelloid” is silent). Biologists have concluded that the bdelloid rotifers may have existed for tens of millions of years without regular sexual reproduction. In general, lack of genetic recombination leads to the buildup of deleterious mutations, so long-term asexual reproduction typically leads to extinction (see Concept 15.6). However, recent studies indicate that bdelloid rotifers may avoid this problem by taking up fragments of genes directly from the environment during the desiccation–rehydration cycle. Such a mechanism allows genetic recombination among individuals in the absence of direct sexual exchange.

Go to MEDIA CLIP 23.4 Rotifer Feeding
PoL2e.com/mc23.4
Ribbon Worms
Ribbon worms (nemerteans) have simple nervous and excretory systems similar to those of flatworms. Unlike flatworms, however, they have a complete digestive tract with a mouth at one end and an anus at the other. Small ribbon worms move slowly by beating their cilia. Larger ones employ waves of muscle contraction to move over the surface of sediments or to burrow into them.
Within the body of nearly all of the 1,200 species of ribbon worms is a fluid-filled cavity called the rhynchocoel, within which lies a hollow, muscular proboscis. The proboscis, which is the worm’s feeding organ, may extend much of the length of the body. Contraction of the muscles surrounding the rhynchocoel causes the proboscis to evert (turn inside out) explosively through an anterior pore (FIGURE 23.14A). The proboscis may be armed with sharp stylets that pierce prey and discharge paralytic toxins into the wound.
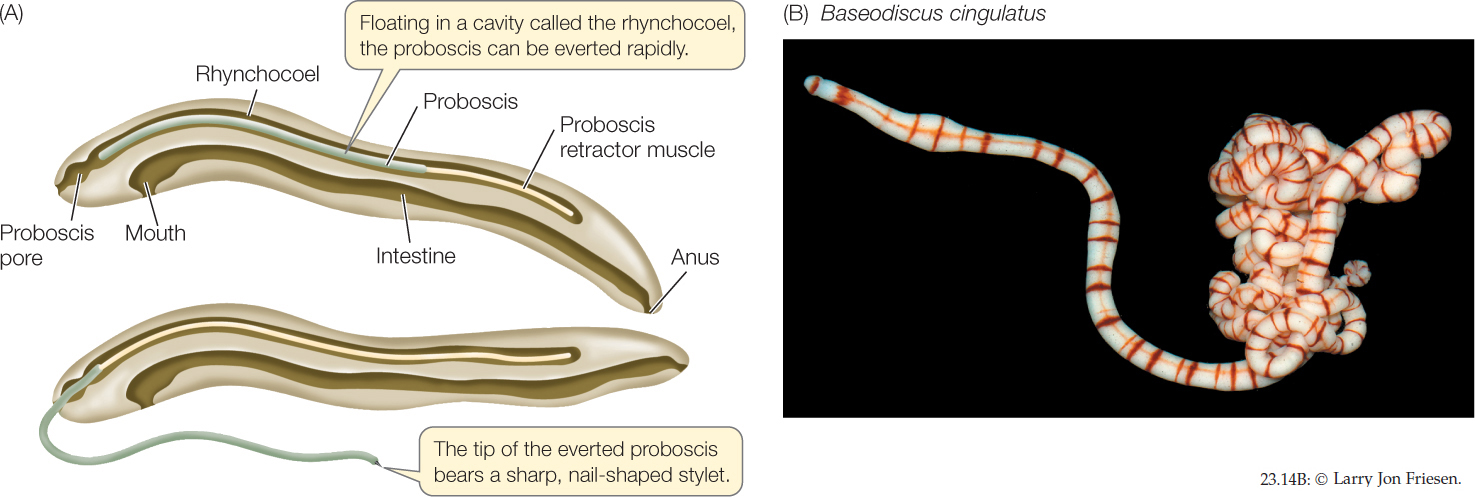
Ribbon worms are largely marine, although there are some freshwater and terrestrial species. Most ribbon worms are less than 20 centimeters long, but individuals of some species reach 20 meters or more. Some genera include species that are conspicuous and brightly colored (FIGURE 23.14B).
483

Go to MEDIA CLIP 23.5 Explosive Extrusion of Ribbon Worm Proboscis
PoL2e.com/mc23.5
Brachiopods and Phoronids
Like bryozoans (see Figure 23.11A), brachiopods and phoronids also feed using a lophophore, but this structure may have evolved independently in these groups. Although neither the brachiopods nor the phoronids are represented by many living species, the brachiopods—which have shells and thus leave an excellent fossil record—are known to have been much more abundant during the Paleozoic and Mesozoic eras.
Brachiopods (lampshells) are solitary marine animals with a rigid shell that is divided into two parts connected by a ligament (FIGURE 23.15). Although brachiopods superficially resemble bivalve mollusks, shells evolved independently in the two groups. The two halves of the brachiopod shell are dorsal and ventral, rather than lateral as in bivalves. The lophophore is located within the shell. Most brachiopods are 4–6 centimeters long. More than 26,000 fossil brachiopod species have been described, but only about 450 species survive.
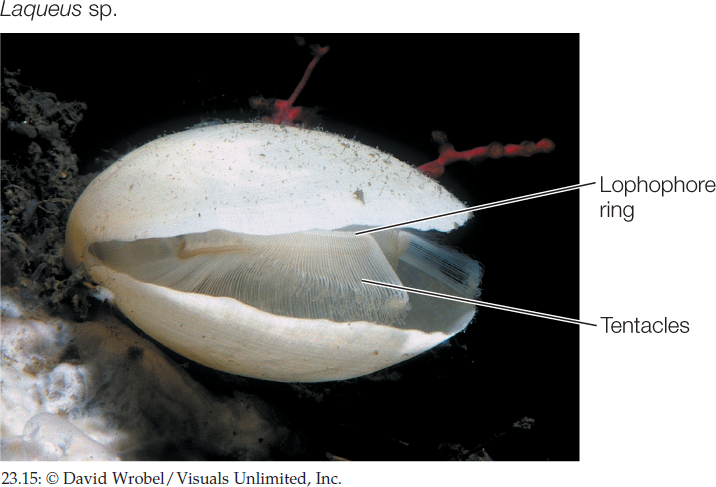
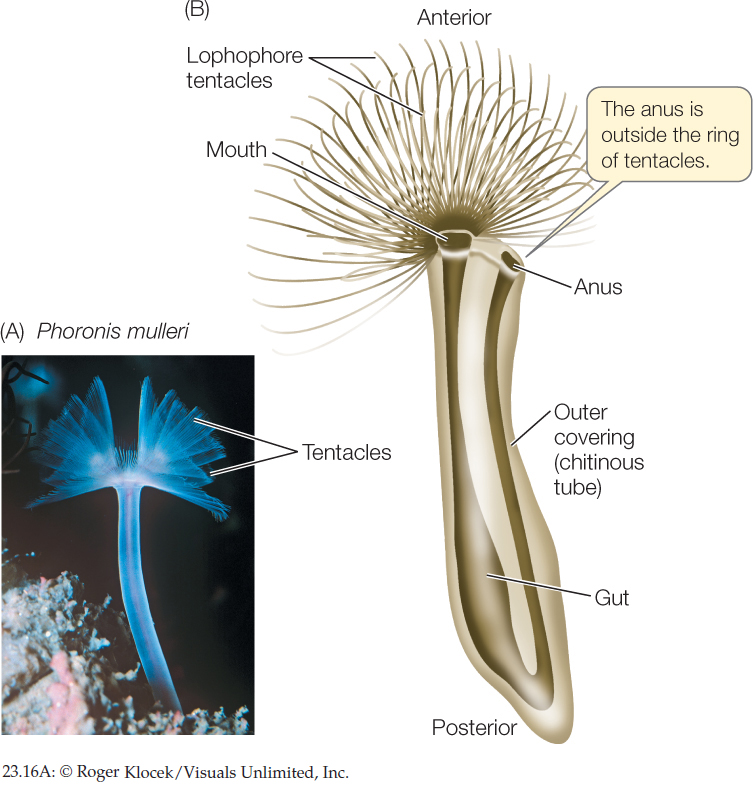
The 10 known species of phoronids are small (5–25 cm long), sessile worms that live in muddy or sandy sediments or attached to rocky substrates. Phoronids are found in marine waters, from the intertidal zone to about 400 meters deep. They secrete tubes made of chitin, within which they live (FIGURE 23.16). Their cilia drive water into the top of the lophophore, and the water exits through the narrow spaces between the tentacles. Suspended food particles are caught and transported to the mouth by ciliary action. Eggs are fertilized internally, and the embryos are either released into the water or, in species with large embryos, retained in the parent’s body, where they are brooded until they hatch.
484
Annelids
As discussed in Concept 23.1, segmentation allows an animal to move different parts of its body independently of one another, giving it much better control of its movement. A clear and obvious example of segmentation is seen in the body plan of the annelids (FIGURE 23.17; see also Figure 23.5A).
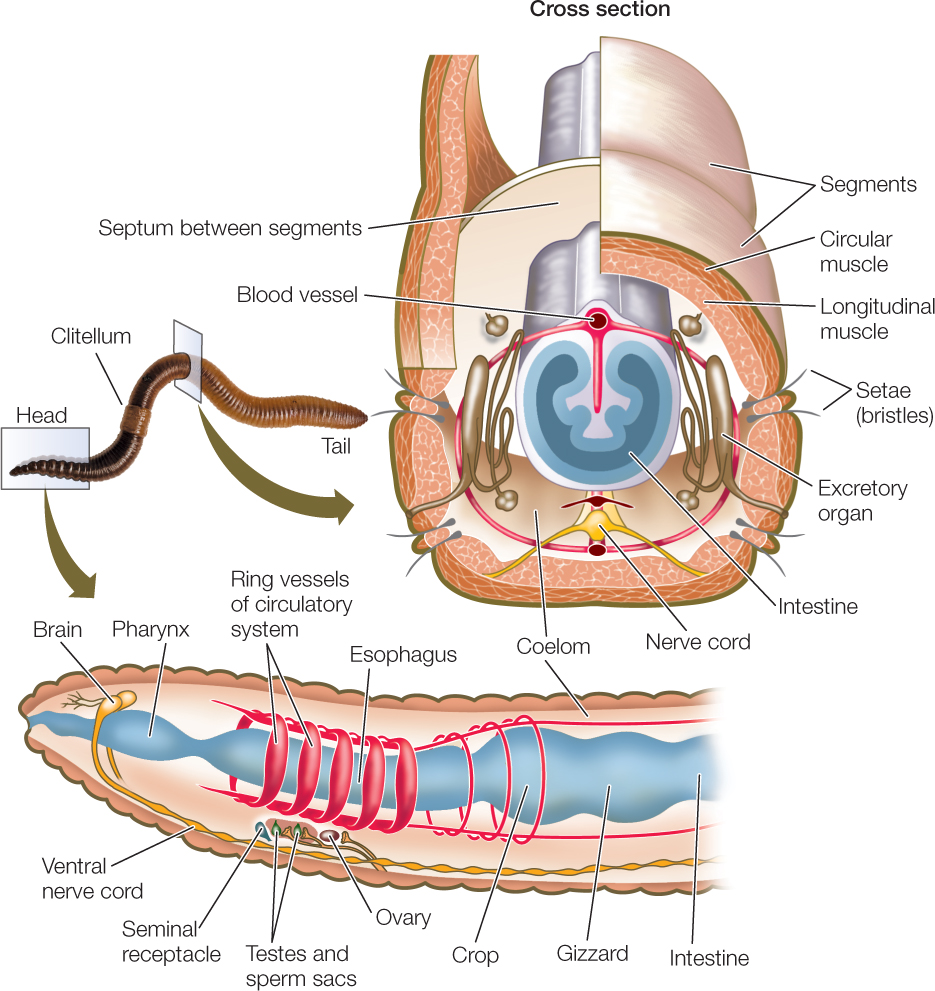
In most large annelids, the coelom in each segment is isolated from those in other segments. A separate nerve center called a ganglion (plural ganglia) controls each segment. Nerve cords that connect the ganglia coordinate their functioning. Most annelids lack a rigid external protective covering. Instead, they have a thin, permeable body wall that serves as a general surface for gas exchange. These animals are thus restricted to moist environments because they lose body water rapidly in dry air. The approximately 19,000 described annelid species live in marine, freshwater, and moist terrestrial environments.
Many annelids have one or more pairs of eyes and one or more pairs of tentacles (with which they capture prey or filter food from the surrounding water) at the anterior end of the body (FIGURE 23.18A). In some species, the body wall of most segments extends laterally as a series of thin outgrowths called parapodia. The parapodia function in gas exchange, and some species use them to move. Stiff bristles called setae protrude from each parapodium, forming temporary contact with the substrate and preventing the animal from slipping backward when its muscles contract.

485
Some annelids, such as the pogonophorans, secrete tubes made of chitin and other substances, in which they live (FIGURE 23.18B). Pogonophorans have lost their digestive tract (they have no mouth or gut). So how do they obtain nutrition? Part of the answer is that pogonophorans can take up dissolved organic matter directly from the sediments in which they live or from the surrounding water. Much of their nutrition, however, is provided by endosymbiotic bacteria that live in a specialized organ known as the trophosome. These bacteria oxidize hydrogen sulfide and other sulfur-containing compounds, fixing carbon from methane in the process. The uptake of the hydrogen sulfide, methane, and oxygen used by the bacteria is facilitated by hemoglobin in the pogonophorans’ tentacles. It is this hemoglobin that gives the tentacles their red color (see Figure 23.18B).
Oligochaetes have no parapodia or anterior tentacles, and they have only four pairs of setae bundles per segment. Earthworms—the most familiar oligochaetes—burrow in and ingest soil, from which they extract food particles. All oligochaetes are hermaphroditic; that is, each individual is both male and female. Sperm are exchanged simultaneously between two copulating individuals. Eggs and sperm are deposited outside the adult’s body, in a cocoon secreted by the clitellum (see Figure 23.17). Fertilization occurs within the cocoon after it is shed, and when development is complete, miniature worms emerge and immediately begin independent life.
Leeches, like oligochaetes, lack parapodia and tentacles (FIGURE 23.18C). They live in freshwater, marine, and terrestrial habitats. The leech coelom is not divided into compartments, and consists of a large, fluid-filled cavity. Groups of segments at each end of the body are modified to form suckers, which serve as temporary anchors that help the leech move. With its posterior sucker attached to a substrate, the leech extends its body by contracting its circular muscles. The anterior sucker is then attached, the posterior one detached, and the leech shortens itself by contracting its longitudinal muscles.
Many leeches feed on vertebrate hosts by making an incision from which blood flows. A feeding leech secretes an anticoagulant into the wound to keep the blood flowing. For centuries, medical practitioners used leeches to treat diseases they believed were caused by an excess of blood or by “bad blood.” Although most leeching practices (such as inserting a leech in a person’s throat to alleviate swollen tonsils) have been abandoned, Hirudo medicinalis (the medicinal leech) is used medically even today to reduce fluid pressure and prevent blood clotting in damaged tissues, to eliminate pools of coagulated blood, and to prevent scarring. The anticoagulants of certain other leech species contain anesthetics and blood vessel dilators and are being studied for possible medical uses.

Go to MEDIA CLIP 23.6 Leeches Feeding on Blood
PoL2e.com/mc23.6
Mollusks
The most diverse group of lophotrochozoans are the mollusks, with about 117,000 species that inhabit a wide array of aquatic and terrestrial environments. There are four major clades of mollusks: chitons, gastropods, bivalves, and cephalopods. Although these groups differ dramatically in morphology, they all share the same three major body components: a foot, a visceral mass, and a mantle (FIGURE 23.19A).
- The molluscan foot is a muscular structure that originally was both an organ of locomotion and a support for internal organs. In cephalopods such as squids and octopuses, the foot has been modified to form arms and tentacles borne on a head with complex sensory organs. In other groups, such as clams, the foot is a burrowing organ. In some groups the foot is greatly reduced.
- The heart and the digestive, excretory, and reproductive organs are concentrated in a centralized, internal visceral mass.
- The mantle is a fold of tissue that covers the organs of the visceral mass. The mantle secretes the hard, calcareous shell that is typical of many mollusks.
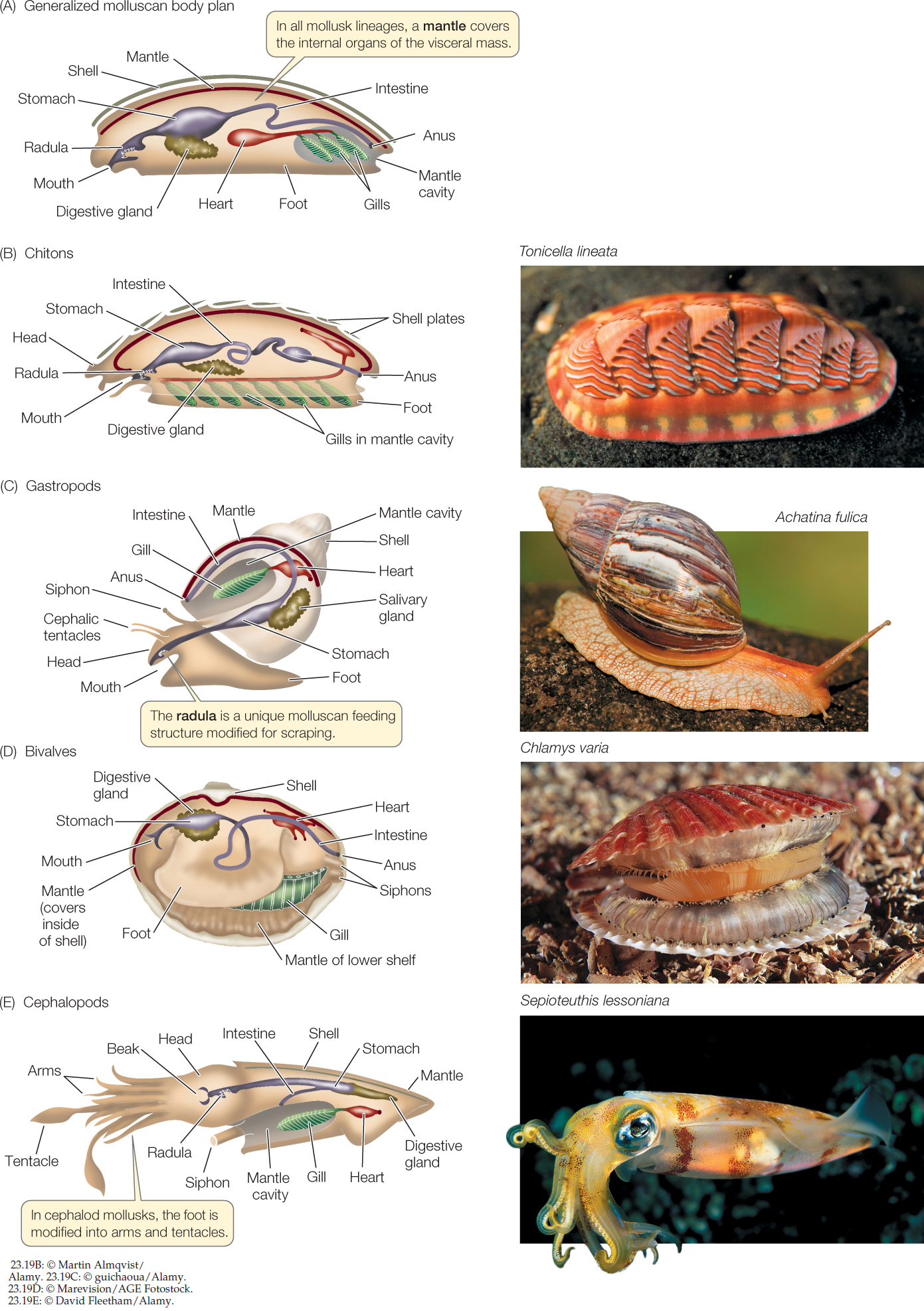 Figure 23.19: Organization and Diversity of Molluscan Bodies (A) The major molluscan groups display different variations on a general body plan that includes three major components: a foot, a visceral mass of internal organs, and a mantle. In many species, the mantle secretes a calcareous shell. (B) Chitons have eight overlapping calcareous plates surrounded by a girdle. (C) Most gastropods have a single dorsal shell, into which they can retreat for protection. (D) Bivalves get their name from their two hinged shells, which can be tightly closed. (E) Cephalopods are active predators; they use their arms and tentacles to capture prey.
Figure 23.19: Organization and Diversity of Molluscan Bodies (A) The major molluscan groups display different variations on a general body plan that includes three major components: a foot, a visceral mass of internal organs, and a mantle. In many species, the mantle secretes a calcareous shell. (B) Chitons have eight overlapping calcareous plates surrounded by a girdle. (C) Most gastropods have a single dorsal shell, into which they can retreat for protection. (D) Bivalves get their name from their two hinged shells, which can be tightly closed. (E) Cephalopods are active predators; they use their arms and tentacles to capture prey.
In most mollusks, the mantle extends beyond the visceral mass to form a mantle cavity. Within this cavity lie gills that are used for gas exchange. When cilia on the gills beat, they create a current of water. The gill tissue, which is highly vascularized (contains many blood vessels), takes up oxygen from the water and releases carbon dioxide. Many mollusks use their gills as filter-feeding devices. Other mollusks feed using a rasping structure known as the radula to scrape algae from rocks. In some mollusks, such as the marine cone snails, the radula has been modified into a drill or poison dart.
Except in cephalopods, molluscan blood vessels do not form a closed circulatory system. Blood and other fluids empty into a large, fluid-filled hemocoel, through which fluids move around the animal and deliver oxygen to the internal organs. Eventually the fluids reenter the blood vessels and are moved by a heart.
The approximately 1,000 living species of chitons (FIGURE 23.19B) are characterized by eight overlapping calcareous plates, surrounded by a structure known as the girdle. These plates and girdle protect the chiton’s internal organs and muscular foot. The chiton body is bilaterally symmetrical, and the internal organs, particularly the digestive and nervous systems, are relatively simple. Most chitons are marine omnivores that scrape algae, bryozoans, and other organisms from rocks with their sharp radula. An adult chiton spends most of its life clinging to rock surfaces with its large, muscular, mucus-covered foot. It moves slowly by means of rippling waves of muscular contraction in the foot. Fertilization in most chitons takes place in the water, but in a few species fertilization is internal and embryos are brooded within the body.
Gastropods are the most species-rich and widely distributed mollusks, with about 85,000 living species. Snails, whelks, limpets, slugs, nudibranchs (sea slugs), and abalones are all gastropods. Most species move by gliding on their muscular foot, but in a few species—the sea butterflies and heteropods—the foot is a swimming organ with which the animal moves through open ocean waters. The only mollusks that live in terrestrial environments—land snails and slugs—are gastropods (FIGURE 23.19C). In these terrestrial species, the mantle tissue is modified into a highly vascularized lung.
486
487
Clams, oysters, scallops, and mussels are all familiar bivalves. The 30,000 living species are found in both marine and freshwater environments. Bivalves have a very small head and a hinged, two-part shell that extends over the sides of the body as well as the top (FIGURE 23.19D). Many clams use their foot to burrow into mud and sand. Bivalves feed by taking in water through an opening called an incurrent siphon and filtering food from the water with their large gills, which are also the main sites of gas exchange. Water and gametes exit through the excurrent siphon. Fertilization takes place in open water in most species.
There are about 800 living species of cephalopods—squids, octopuses, and nautiluses. Their excurrent siphon is modified to allow the animal to control the water content of the mantle cavity. The modification of the mantle into a device for forcibly ejecting water from the cavity through the siphon enables these animals to move rapidly by “jet propulsion” through the water. With their greatly enhanced mobility, cephalopods (which first appeared early in the Cambrian) became the major predators in the open waters of the Devonian oceans. They remain important marine predators today.
Cephalopods capture and subdue prey with their tentacles (see Figure 23.20B). As is typical of active, rapidly moving predators, cephalopods have a head with complex sensory organs, most notably eyes that are comparable to those of vertebrates in their ability to resolve images. The head is closely associated with a large, branched foot that bears the tentacles and a siphon (FIGURE 23.19E). The large, muscular mantle provides an external supporting structure. The gills hang in the mantle cavity. Many cephalopods have elaborate courtship behavior, which can involve striking color changes.
Many early cephalopods had a chambered external shell divided by partitions penetrated by tubes through which gases and liquids could be moved to control the animal’s buoyancy. Nautiluses are the only surviving cephalopods that have such external chambered shells, although squids and cuttlefishes retain internal shells.
Shells have been lost several times among the mollusks, as in several groups of gastropods, including the slugs and nudibranchs (FIGURE 23.20A). These shell-less gastropods gain some protection from predation by being distasteful or toxic to many species. The often brilliant coloration of nudibranchs is aposematic, meaning it serves to warn potential predators of toxicity. Among the cephalopods, the octopuses have lost both external and internal shells (FIGURE 23.20B). Their lack of shells allows octopuses to escape predators by squeezing into small crevices.


Go to MEDIA CLIP 23.7 Octopuses Can Pass through Small Openings
PoL2e.com/mc23.7
Ecdysozoans must shed their cuticles
The distinguishing characteristic of ecdysozoans is their external covering, or cuticle, which is secreted by the underlying epidermis (the outermost cell layer). The cuticle provides these animals with both protection and support. Once formed, however, the cuticle cannot grow. How, then, can ecdysozoans increase in size? They do so by shedding, or molting, the cuticle and replacing it with a new, larger one. This molting process gives the clade its name (Greek ecdysis, “to get out of”).

Go to MEDIA CLIP 23.8 Molting a Cuticle
PoL2e.com/mc23.8
A soft-bodied arthropod from the Cambrian, fossilized in the process of molting, shows that molting evolved more than 500 million years ago (FIGURE 23.21A). An increasingly rich array of molecular and genetic evidence, including a set of Hox genes shared by all ecdysozoans, suggests they have a single common ancestor. Thus molting of a cuticle is a trait that may have evolved only once during animal evolution.
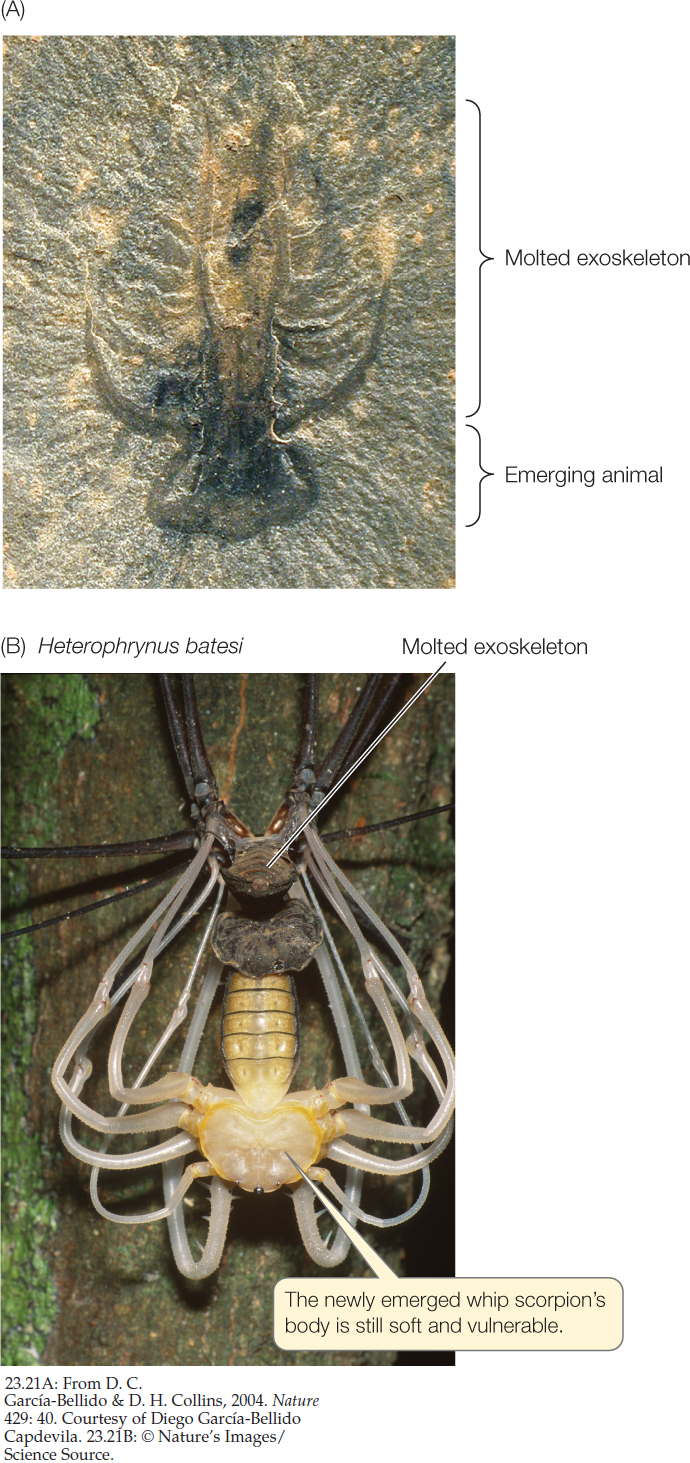
Before an ecdysozoan molts, a new cuticle is already forming underneath the old one. Once the old cuticle is shed, the new one expands and hardens. Until it has hardened, though, the animal is vulnerable to its enemies, both because its outer surface is easy to penetrate and because an animal with a soft cuticle moves slowly or not at all (FIGURE 23.21B).
488
In many ecdysozoans that have wormlike bodies, the cuticle is relatively thin and flexible. Such a cuticle offers the animal some protection but provides only modest body support. A thin cuticle allows the exchange of gases, minerals, and water across the body surface, but it restricts the animal to moist habitats. Many species of ecdysozoans with thin cuticles live in marine sediments from which they obtain prey. Some freshwater species absorb nutrients directly through their thin cuticles, as do parasitic species that live within their hosts.
The cuticles of other ecdysozoans, notably the arthropods, function as external skeletons, or exoskeletons. These exoskeletons are thickened by layers of protein and a strong, waterproof polysaccharide called chitin. An animal with a rigid, chitin-reinforced exoskeleton can neither move in a wormlike manner nor use cilia for locomotion. A hard exoskeleton also impedes the passage of oxygen and nutrients into the animal, presenting new challenges in other areas besides growth. New mechanisms of locomotion and gas exchange evolved in those ecdysozoans with hard exoskeletons.
To move rapidly, an animal with a rigid exoskeleton must have body extensions that can be manipulated by muscles. Such appendages evolved in the late Precambrian and led to the arthropod (Greek, “jointed foot”) clade. Arthropod appendages exist in an amazing variety of forms. They serve many functions, including walking and swimming, gas exchange, food capture and manipulation, copulation, and sensory perception. Arthropods grasp food with their mouths and associated appendages and usually digest it internally. Their muscles are attached to the inside of the exoskeleton.
The arthropod exoskeleton has had a profound influence on the evolution of these animals. Encasement within a rigid body covering provides support for walking on dry land, and the waterproofing provided by the cuticle keeps the animal from dehydrating in dry air. These features have allowed arthropods to invade terrestrial environments several times.
The evolution of the diverse arthropods and their relatives will be described in greater detail in Concept 23.4. Here we describe the other major groups of ecdysozoans.
Nematodes
Nematodes (roundworms) have a thick, multilayered cuticle that gives their unsegmented body its shape. As a nematode grows, it sheds its cuticle four times. Nematodes exchange oxygen and nutrients with their environment through both the cuticle and the gut, which is only one cell layer thick. Materials are moved through the gut by rhythmic contraction of a highly muscular organ, the pharynx, at the worm’s anterior end. Nematodes move by contracting their longitudinal muscles.
Nematodes are probably the most abundant and universally distributed of all major animal groups. About 25,000 species have been described, but the actual number of living species may be in the millions. Many are microscopic, but the largest known nematode reaches a length of 9 meters (it is a parasite in the placentas of sperm whales). Countless nematodes live as scavengers in the upper layers of the soil, on the bottoms of lakes and streams, and in marine sediments. The topsoil of rich farmland may contain 3–9 billion nematodes per acre. A single rotting apple may contain as many as 90,000 individuals.
One soil-inhabiting nematode, Caenorhabditis elegans, serves as a model organism in the laboratories of many geneticists and developmental biologists. It is ideal for such research because it is easy to cultivate, matures in 3 days, and has a fixed number of body cells.
489
Many nematodes are predators, feeding on protists and small animals (including other roundworms). Most significant to humans, however, are the many species that parasitize plants and animals (FIGURE 23.22A). The nematodes that parasitize humans (causing serious diseases such as trichinosis, filariasis, and elephantiasis), domesticated animals, and economically important plants have been studied intensively in an effort to find ways of controlling them.
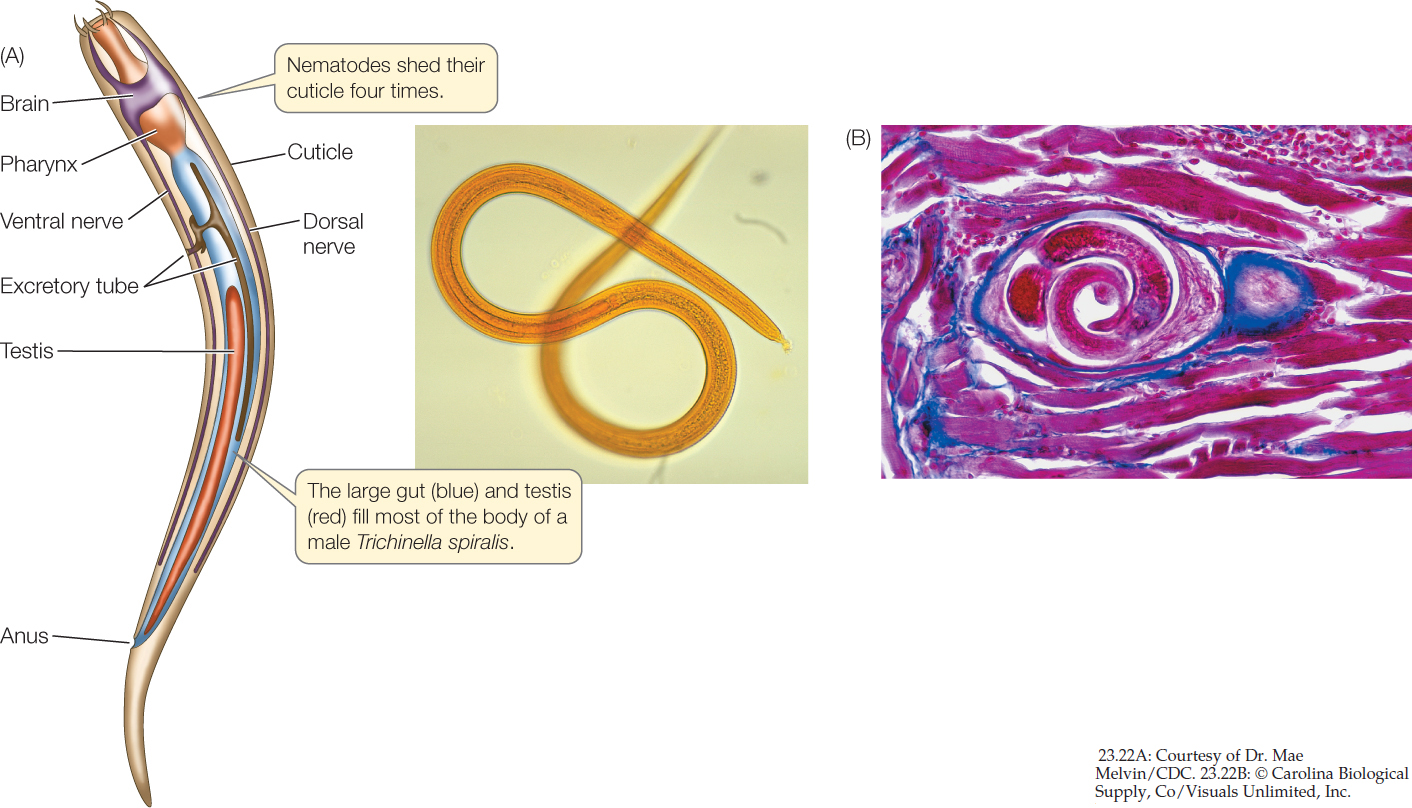
The structure of parasitic nematodes is similar to that of free-living species, but the life cycles of many parasitic species have special stages that facilitate the transfer of individuals among hosts. Trichinella spiralis, the species that causes the human disease trichinosis, has a relatively simple life cycle. A person may become infected by eating the flesh of an animal (usually a pig) that has Trichinella larvae encysted in its muscles (FIGURE 23.22B). The larvae are activated in the person’s digestive tract, emerge from their cysts, and attach to the intestinal wall, where they feed. Later they bore through the intestinal wall and are carried in the bloodstream to muscles, where they form new cysts. If present in great numbers, these cysts can cause severe pain or death.
Horsehair Worms
About 350 species of the unsegmented horsehair worms have been described. As their name implies, these animals are extremely thin in diameter; they range from a few millimeters up to a meter in length. Most adult horsehair worms live in fresh water among the leaf litter and algal mats that accumulate near the shores of streams and ponds. A few species live in damp soil.
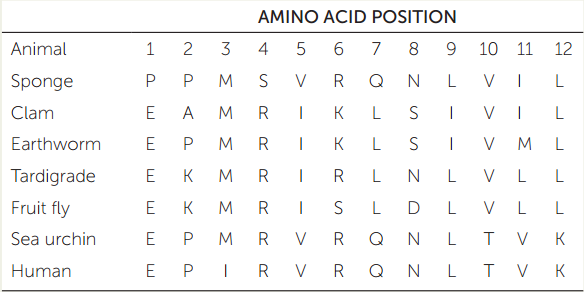
APPLY THE CONCEPT: There are two major groups of protostomes
The table shows a small sample of amino acid residues that have been used to reconstruct the relationships of some major animal groups. (The full dataset includes data on 11,234 amino acid positions across 77 species of animals.)
- Construct a phylogenetic tree of these seven animals using the parsimony method (see Chapter 16). Use the sponge as your outgroup to root the tree. Assume that all changes among amino acids are equally likely.
- How many changes (from one amino acid residue to another) occur along each branch on your tree?
- Which branch on your tree represents the eumetazoans?
- The protostomes? The lophotrochozoans? The ecdysozoans? The deuterostomes?
490
Horsehair larvae are internal parasites of freshwater crayfishes and of terrestrial and aquatic insects (FIGURE 23.23). An adult horsehair worm has no mouth, and its gut is greatly reduced and probably nonfunctional. Some species feed only as larvae, absorbing nutrients from their hosts across the body wall. But other species continue to grow and shed their cuticles even after they have left their hosts, suggesting that some adult worms may be able to absorb nutrients from their environment.

Small Marine Clades
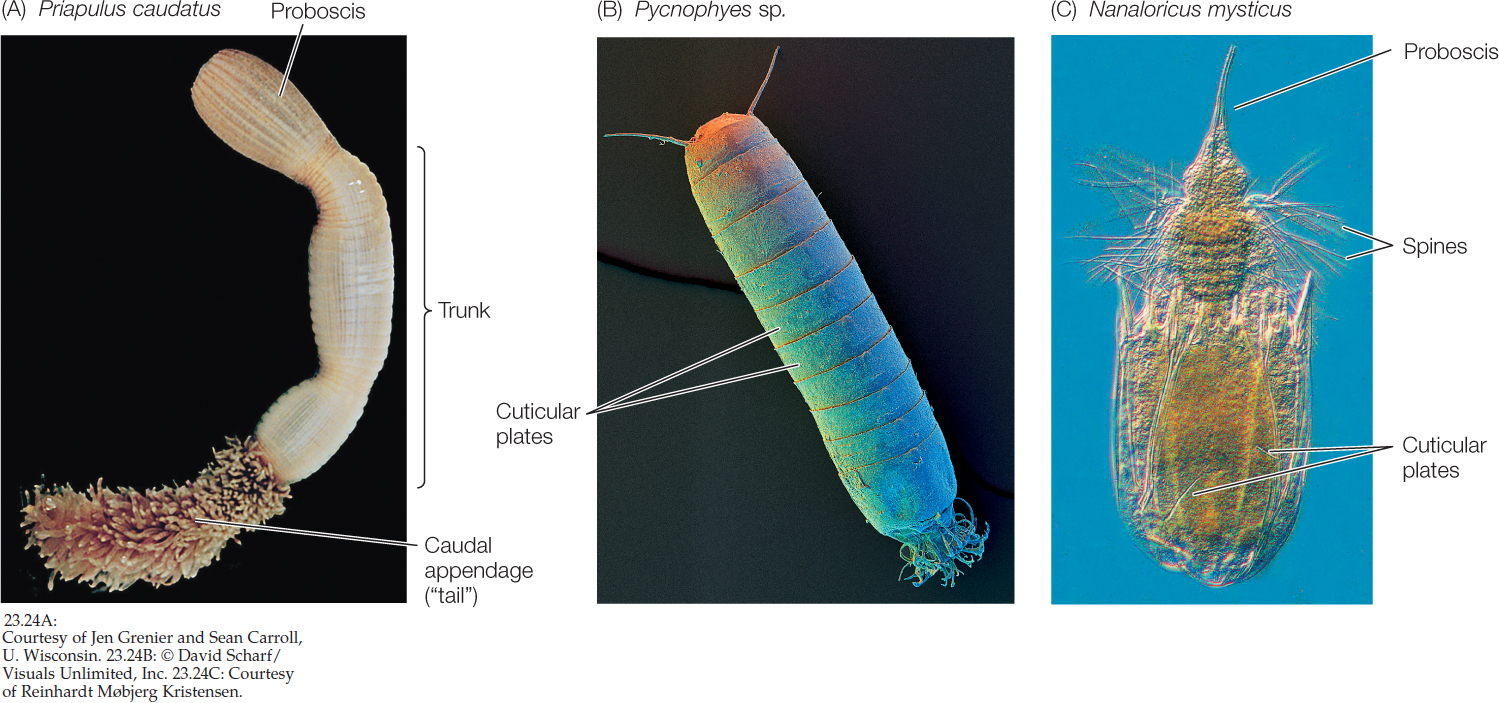
There are several small, relatively poorly known groups of benthic marine ecdysozoans. The 20 species of priapulids are cylindrical, unsegmented, wormlike animals with a three-part body plan consisting of a proboscis, trunk, and caudal appendage (“tail”). It should be clear from their appearance why they were named after the Greek fertility god Priapus (FIGURE 23.24A). The 180 species of kinorhynchs live in marine sands and muds and are virtually microscopic; no kinorhynchs are longer than 1 millimeter. Their bodies are divided into 13 segments, each with a separate cuticular plate (FIGURE 23.24B). The minute (less than 1 mm long) loriciferans were not discovered until 1983. About 100 living species are known to exist, although only about 30 species have been described. The body is divided into a head, neck, thorax, and abdomen and is covered by six plates, from which the loriciferans get their name (Latin lorica, “corset”; FIGURE 23.24C).
CHECKpoint CONCEPT 23.3
- How does an animal’s body covering influence the way it breathes, feeds, and moves?
- Why are most annelids restricted to moist environments?
- Briefly describe how the basic body organization of mollusks has been modified to yield a wide diversity of animals.
- What are three ways in which nematodes have a significant impact on humans?
We will turn now to the arthropods. Members of the four arthropod subgroups not only dominate the ecdysozoan clade but are also among the most diverse animals on Earth.
491