Concept 30.1: Food Provides Energy and Chemical Building Blocks
Animals—being heterotrophs (see Concept 29.1)—obtain their energy and chemical building blocks from eating other organisms. For animals in the wild, this process is frequently dangerous. For example, Thomas Ruf, Claudia Bieber, and Christopher Turbill recently studied various species of mammals that hibernate, asking whether individuals have different death rates when they are hibernating and not hibernating. When an individual is hibernating, it remains continually in an underground burrow, out of sight. When the same individual is awake, its metabolic rate is far higher and it has to venture out of its burrow to collect food. Ruf and his colleagues found that, on average, the odds of dying each month are five times higher when an individual is awake rather than hibernating. They attributed this difference to the dangers of getting food. As modern humans in an industrialized society, we may have a hard time understanding this. When we need food, we go to a supermarket, and maybe have an ice-cream treat on our way. For animals in the wild, getting food is risky. Plant eaters can be seen by predators and possibly attacked while they munch on grass or leaves. Meat eaters face the very real risk that animals they attack may fight back and injure them, possibly leading to blood loss or infection.
Eating must be of great importance for animals to risk their lives to get food. What exactly do animals require from their food? We discussed these needs briefly in Chapter 29. First, because animals are heterotrophs, they require chemical-bond energy to live: they need organic molecules that they can break down to release energy that they can then use to move about, pump blood, synthesize body parts, and carry out the other energy-demanding requirements of life. Second, animals eat because they require chemical building blocks. They need materials that they can use to build the parts of their bodies—such as amino acids to build proteins, fatty acids to build cell membranes, and calcium to build a skeleton. This need for chemical building blocks is far more complicated than you might imagine, because animals often cannot synthesize for themselves certain molecules they require for life. They need to get those specific molecules from food.
Food provides energy
We quantify the energy content of a food by measuring the amount of heat the food produces when it is completely burned in the presence of oxygen, producing CO2, H2O, and other fully oxidized chemical products (FIGURE 30.1). We can determine the energy content of a food this way because both burning and aerobic animal respiration follow the same chemical rules. Burning takes place by different chemical steps than respiration, but the products of the two processes are almost identical. Heat is one product. Therefore the amount of heat released by burning a food is close to the same as that produced when an animal metabolically breaks down the same food (and we can correct for any differences by use of chemical rules).
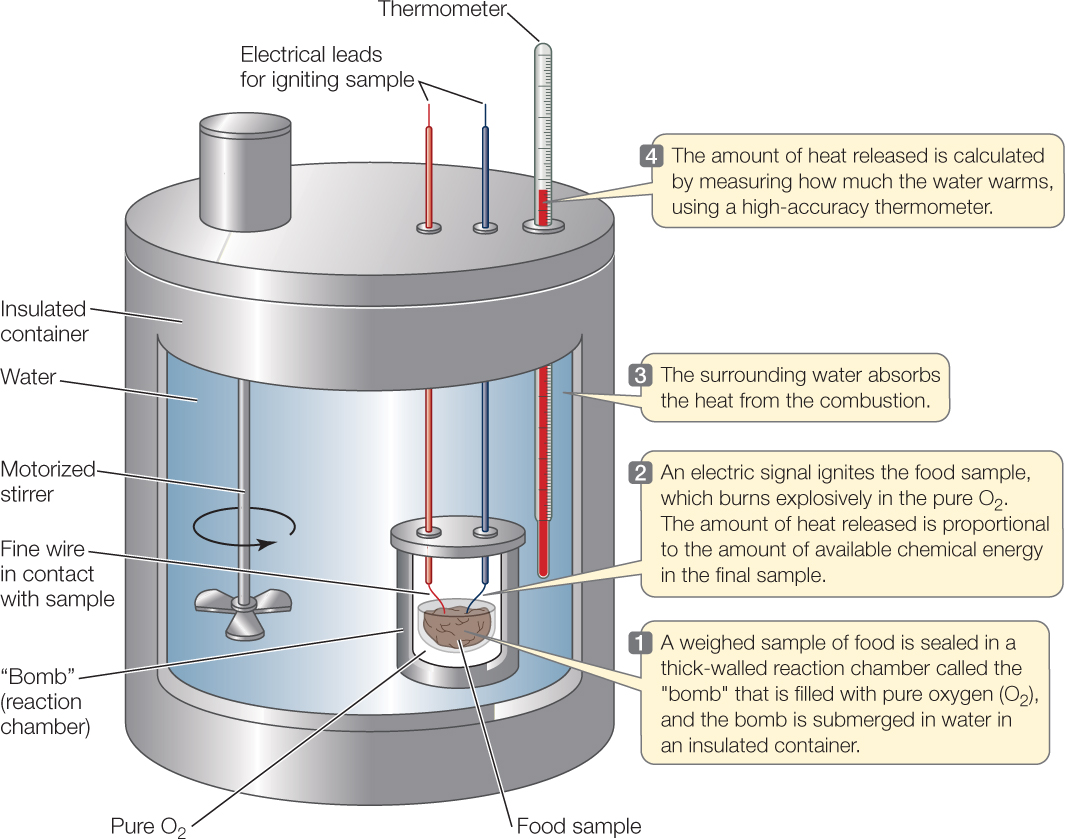
Food energy values in the United States are typically expressed in calories and kilocalories. A calorie (cal) is the amount of heat required to raise the temperature of 1 gram of water by 1°C, and a kilocalorie (kcal) is 1,000 times greater. We can use these units for any type of energy, not just heat, because all forms of energy are proportional to one another.
Confusion arises because people often refer to kilocalories as “calories” in everyday life. When you see the word “calories,” you should check whether calories or kilocalories are being used. In the Nutrition Facts on food packages in the United States, for example, the energy values reported as “calories” are actually kilocalories (FIGURE 30.2). The joule is an alternative, metric unit for measuring energy (1 calorie = 4.2 joule). Most countries in the world use the joule, and scientists almost always use it in their scientific work.
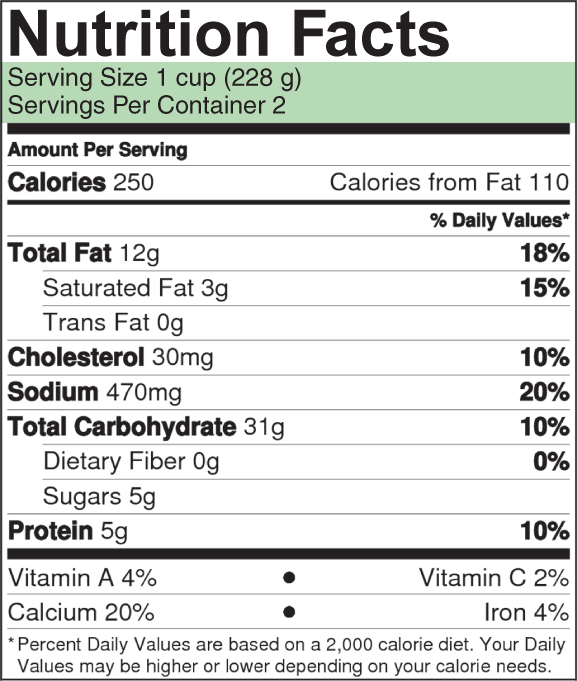
Let’s consider a meal of macaroni and cheese for which the Nutrition Facts are presented in Figure 30.2. As you can see, each serving supplies 250 kcal. When the meal is sitting on your plate waiting to be eaten, this energy is in the form of chemical energy—in the chemical bonds of the starch, fats, and other molecules in the meal. After you’ve eaten the macaroni and cheese, if your body breaks down all the molecules in it to release their energy, your body will produce 250 kcal of heat as it uses the energy.
626
How much chemical energy does an animal consume each day, converting the chemical energy to heat? Metabolic rate provides the answer (see Concept 29.2). An animal’s metabolic rate, expressed per day, is the amount of energy the animal converts to heat per day. What if we focus specifically on people? A rule of thumb is that adults in modern society use an average of 2,000–2,500 kcal per day in their daily mix of rest and activity. For general purposes, you can be sure you don’t overeat and gain weight by adding up the energy values of all the foods you eat each day and making sure the total is no more than 2,000–2,500 kcal.
There are three major types of food molecules: lipids (fats and oils), carbohydrates, and proteins. Lipids are about twice as dense with energy as carbohydrates and proteins. Lipids yield about 9.4 kcal per gram when they are broken down, compared with 4.1 kcal/g for carbohydrates and 4.5 kcal/g for proteins. As you might expect, foods containing lots of lipids tend to provide lots of kilocalories per serving.
Chemical energy from food is sometimes stored for future use
When an animal eats foods containing more chemical energy than the animal needs, the animal often stores some or all of the extra chemical energy for future use. Animals store energy mostly in the form of lipids. This makes sense because lipids are particularly energy dense. Stored materials need to be carried around until they are used. For each gram of lipid that an animal stores and carries, it gets more than 9 kcal of energy—a large amount—when it finally uses the lipid. Animals do not need to eat lipids to store lipids. They can convert carbohydrates and proteins to lipids to store chemical energy.
Animals also store glycogen, a type of carbohydrate (specifically, polymerized glucose), but they do not store nearly as much energy as glycogen as they store as lipid. Glycogen is always stored with a lot of water bound to its molecules. When all this water is taken into account, the glycogen is heavy, and its energy density is only about 1 kcal/g.
Why do animals store glycogen at all if its energy value is only 1 kcal/g compared with more than 9 kcal/g for lipids? The answer is that some tissues require carbohydrates as their energy source. When glycogen is broken down, it yields the sugar glucose. The vertebrate brain gets its energy almost entirely from glucose. Thus glycogen stores can directly meet the brain’s energy needs. When skeletal muscles employ anaerobic glycolysis to make ATP for exercise (see Concept 29.4), they also need glucose as their energy source.
APPLY THE CONCEPT: Food provides energy and chemical building blocks
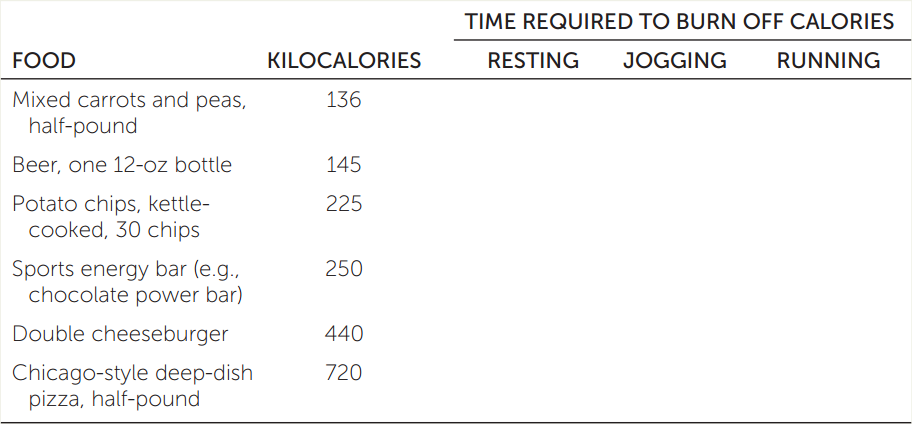
MET, the metabolic equivalent of task, is an index of the intensity of physical activity. Exercise physiologists define the resting metabolic rate as 1 MET and increasingly energetic activities as multiples of 1 MET. Walking at 4 miles per hour has a value of about 3 METs (i.e., it uses 3 times as much energy per unit of time as resting quietly). Jogging (7 mph) requires about 8 METs, and vigorous running (10 mph) about 12 METs. Assuming that your resting metabolic rate is 80 kcal/hr, fill in the table with the time it would take you to burn off the calories from the listed foods while resting, jogging, or running.
627
Proteins are not usually used as storage compounds. There are large masses of protein in an animal’s body. Muscles are principally proteins, for example. However, these proteins are in use, rather than being stored for future use.
Food provides chemical building blocks
The molecules in an animal’s body are made from the molecules in the animal’s food. For carrying out this process, animals have enormous capacities to interconvert molecules. Lipid molecules can be converted to carbohydrate molecules, and certain amino acids can be converted to others, for example. These are just two of the many interconversion processes that occur in animals.
As an animal grows, its food must meet both its energy needs and its needs for chemical building blocks to form a larger and larger body. The need for chemical building blocks does not end with adulthood, however. As stressed in Concept 29.1, adult animals constantly rebuild their bodies. Mammals, for example, rebuild 2–3 percent of the protein molecules in their bodies every day. Much of this rebuilding is carried out by recycling building blocks within the body. Recycling is not perfect, however. For this reason, adults have a steady need throughout their lives for new building blocks that they obtain by eating.
LINK
For more on the reactions that interconvert macromolecules and their constituent monomers, see Concept 2.2, Concept 2.3, Concept 2.4, Concept 3.1, and Concept 3.2
Some nutrients in foods are essential
The word “essential” has a particular meaning in the study of nutrition. An essential nutrient is one that an animal cannot synthesize for itself. We will start our discussion of essential nutrients by focusing on the standard amino acids: the 20 kinds of amino acids that animals use to build proteins (see Concept 3.2). These amino acids exemplify the distinction between essential and nonessential nutrients.
Biochemists have discovered that animals can synthesize some of the 20 standard amino acids but not others. Adult humans, for example, can synthesize 12 of the standard amino acids by rearranging the subparts (carbon skeletons and functional groups) of other molecules in their diet. Those 12 amino acids are nonessential. The remaining eight amino acids cannot be synthesized by adult humans. These essential amino acids—isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine—must be obtained fully formed in the diet. The list of essential amino acids tends to be similar from one type of animal to another, but there are important differences. Infant humans, for example, require histidine in their diet as well as the eight amino acids that are essential for adults.
You might wonder: Where do the essential amino acids come from? Plants and algae can synthesize all 20 standard amino acids. For this reason, animals can obtain their essential amino acids by eating plants or algae. Different plants and algae vary in their proportions of amino acids. For an animal to get all of its essential amino acids in adequate quantities, it may therefore need to eat more than one kind of plant or alga. For adult humans, combinations of grain foods and legume foods—such as corn and beans—are often effective in providing all the essential amino acids (FIGURE 30.3).
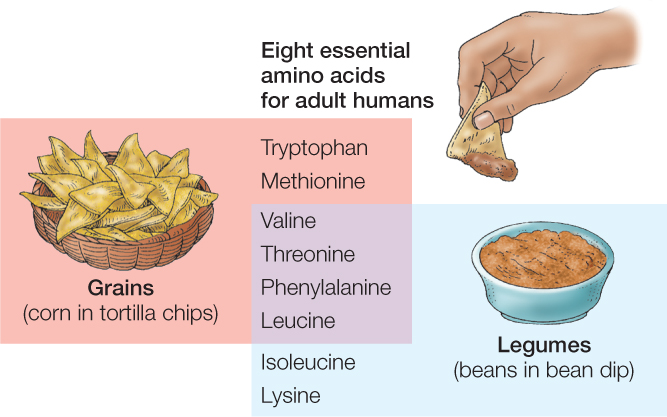
Foods of animal origin—such as milk, eggs, and meat—tend to be good sources of all eight essential amino acids. Cows, chickens, and other farm animals get their essential amino acids by eating plants. They build those amino acids into proteins in their bodies or in the products they make, such as milk and eggs. We then get the amino acids when we eat them.
Animals also need certain essential fatty acids. There are a great many kinds of fatty acids, but in humans and many other animals only two are commonly essential: alpha-linolenic (α-linolenic) acid and linoleic acid. If α-linolenic acid is essential for an animal, the animal must either eat it in its diet or eat very similar fatty acids that can be converted to it by simple reactions. Alpha-linolenic acid and these other fatty acids are called the omega-3 fatty acids. Linoleic acid and other fatty acids that are closely similar to it are the omega-6 fatty acids.
Vitamins are a third group of essential nutrients. Vitamins are difficult to define chemically. This is true because needs for various vitamins have evolved independently or relatively independently, meaning that different vitamins often have unrelated chemical structures. Vitamin A provides an excellent illustration of the evolutionary history of a vitamin:
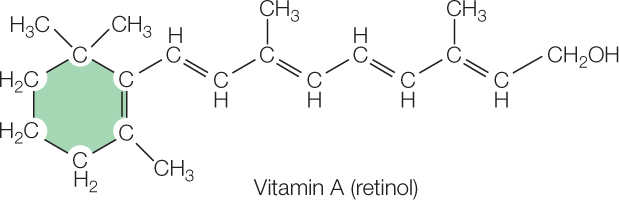
628
When animals evolved vision, they used a slightly modified version of this complex structure for light collection in their eyes. But they did not evolve an ability to make the structure! Instead, they depended on getting the structure, already made, from plants and algae that synthesize the structure and incorporate it into pigments (e.g., β-carotene) that help collect light for photosynthesis. To this day, humans and many other animals must obtain vitamin A as a fully formed (“prefabricated”) chemical structure in their diets because they cannot synthesize it themselves, yet they require it for vision and certain other functions.
All the vitamins are carbon compounds, and they are needed in just tiny amounts. Thus we can quickly define a vitamin as an essential carbon compound required in tiny amounts. TABLE 30.1 summarizes the 13 vitamins commonly recognized as required by humans. Note that vitamins fall into two groups: those that are soluble in water and those that are soluble in lipids. The list of vitamins varies a bit among animals. For example, most mammals can make their own ascorbic acid, so it is not a vitamin for them. However, primates—including humans—cannot synthesize ascorbic acid, and for them it is a vitamin (vitamin C). Vitamin D is a special case because humans can synthesize it if their skin is exposed to sunlight. Vitamin D is essential for people only if they have inadequate sun exposure, as is often true in winter when people stay indoors or wear heavy clothing.
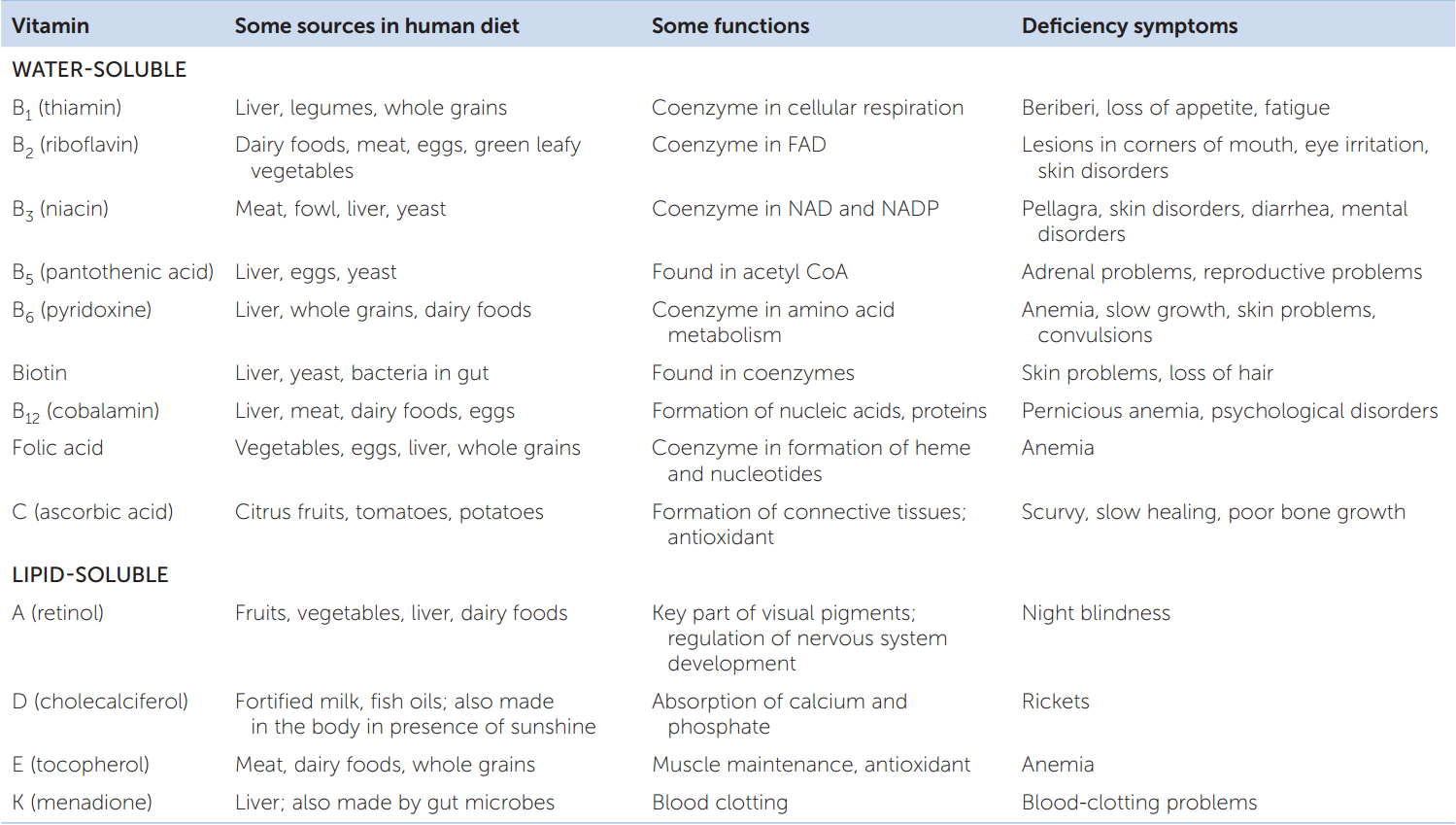
Go to ACTIVITY 30.1 Vitamins in the Human Diet
PoL2e.com/ac30.1
The final category of essential nutrients is the essential minerals. These are chemical elements that animals require in addition to carbon, oxygen, hydrogen, and nitrogen (the most abundant elements in organic molecules). Familiar examples of essential minerals are calcium, iodine, and iron (TABLE 30.2).
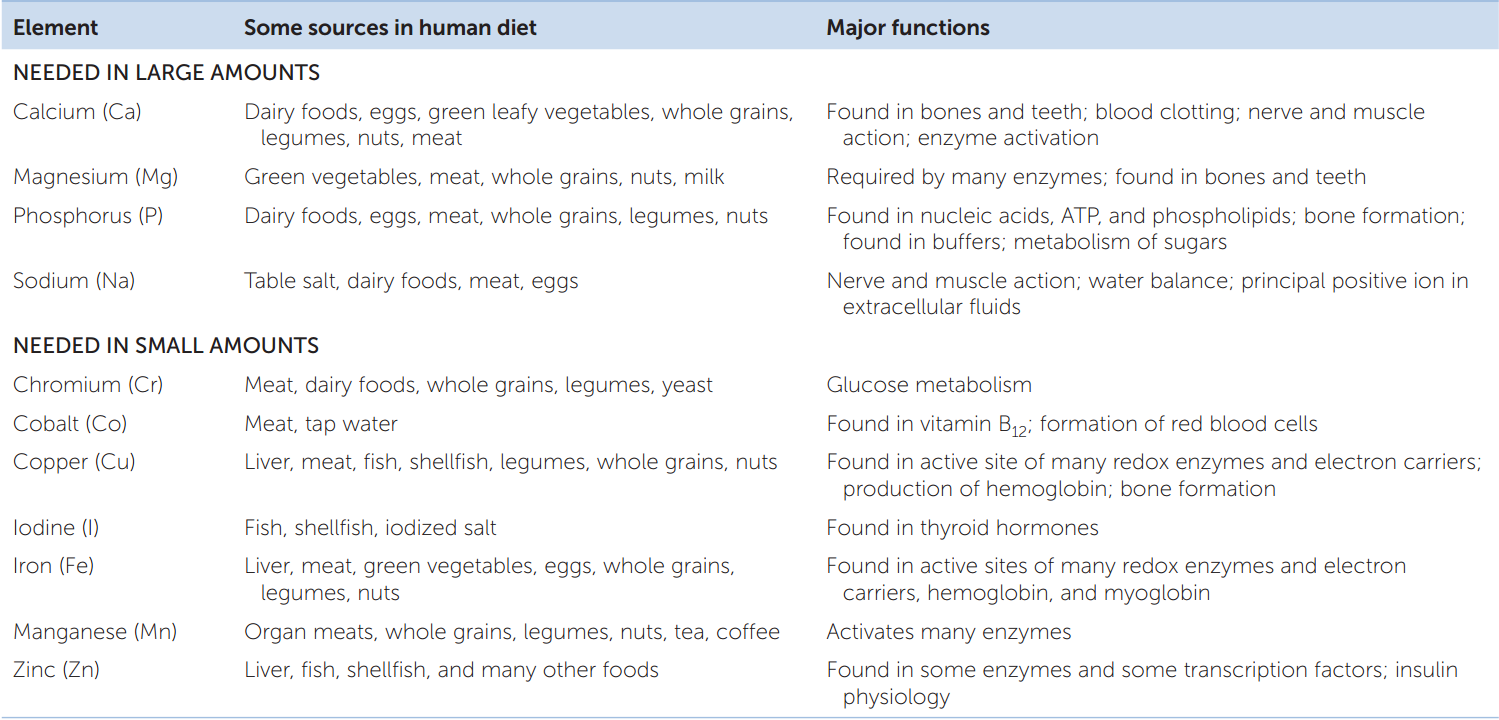
We need some essential minerals in substantial quantities. Calcium, for example, is the fifth most abundant element in the human body. A 70-kg person contains about 1.2 kg of calcium. Calcium is a component of calcium phosphate, the principal structural material in bones and teeth, and is also necessary for nerve function and muscular contraction (see Concept 33.1 and Concept 34.3). We lose calcium from our bodies in urine, sweat, and feces, so it must be replaced regularly. Humans require about 1 g of calcium per day in their diet.
We require some of the other essential minerals in only small amounts. Iron is one of these. It is the oxygen-binding atom in hemoglobin and myoglobin (see Concept 32.5) and is also a component of enzymes in the electron transport chain. Yet the total amount of iron in the tissues of a 70-kg person is only about 4 g. Only tiny amounts (10–20 mg) are required in the diet each day to replace iron lost from the tissues.
Go to ACTIVITY 30.2 Mineral Elements Required by Animals
PoL2e.com/ac30.2
Nutrient deficiencies result in diseases
The lack of any essential nutrient in an animal’s diet produces a state of deficiency called malnutrition. When malnutrition is chronic, it leads to a specific deficiency disease related to the missing nutrient. Scurvy, for example, is a deficiency disease caused by inadequate amounts of a vitamin (see Table 30.1). This complex illness has many debilitating symptoms, including bleeding gums, and is ultimately fatal if not cured. Scurvy was a major problem for sailors at sea, who often had no fresh foods during long trips, until physicians learned in the eighteenth century that it is caused by deficiency of a nutrient, later identified as vitamin C. The disease disappeared when sailors made a point of eating limes or other citrus fruits that provide ample vitamin C.
629
LINK
Deficiencies of essential minerals—for example, iodine—can also cause disease; see Concept 35.4
CHECKpoint CONCEPT 30.1
- Imagine that a slice of pizza contains 300 kcal of energy. Using only the energy stored in the pizza, what is the maximum amount of water you could heat by 1°C? (Hint: Start by converting kilocalories to calories and express your final answer in grams.)
- Which of these food molecules will produce the most heat per gram if burned in the presence of oxygen: lipids, carbohydrates, or proteins?
- Do all organisms require the same essential amino acids?
Now that we have discussed why animals require food, let’s turn to the methods they use to get food.