Concept 32.5: The Blood Transports O₂ and CO₂
Blood consists of cells suspended in a solution called plasma. The plasma typically contains many dissolved materials (solutes), such as glucose and other nutrient molecules, ions, waste products, hormones, and clotting proteins. In vertebrates, most of the cells suspended in the plasma are red blood cells, also called erythrocytes, which contain hemoglobin and carry O2. The blood of vertebrates also contains a smaller number of white blood cells or leukocytes—cells of the immune system—as well as platelets (pinched-off fragments of cells), which are involved in blood clotting.
LINK
The functions of leukocytes in the immune system are discussed in Chapter 39
The most important function of the blood in most types of animals is to transport the respiratory gases, O2 and CO2. Blood proteins that combine with O2, called respiratory pigments, have evolved to increase the amount of O2 each milliliter of blood can carry. O2 is not very soluble in aqueous (watery) solutions such as plasma. For this reason, a respiratory pigment is typically essential for the blood to carry a significant amount of O2. Human blood in the lungs can dissolve only about 4 milliliters of O2 in each liter of blood. Yet the blood actually carries about 200 milliliters of O2 per liter as it leaves the lungs. The respiratory pigment in our blood (hemoglobin) is responsible for the difference.
When a respiratory pigment combines with O2, the process is called oxygenation, and the pigment is said to be oxygenated. This choice of words is important. A respiratory pigment is not chemically oxidized when it combines with O2, so the process is termed oxygenation, not oxidation. When a pigment releases O2, the process is deoxygenation, and the pigment becomes deoxygenated.
In addition to transporting O2, the blood also transports CO2. When CO2 is added to the blood, it reacts with water in the blood to form bicarbonate ions (HCO3−): CO2 + H2O ⇌ H+ + HCO3−. Accordingly, most CO2 is carried as bicarbonate. This bicarbonate can be lost across the gills into the environmental water in aquatic animals. In terrestrial animals, however, the bicarbonate is converted back to CO2 in the lungs and CO2 is exhaled.
Hemoglobin and hemocyanin are the two principal respiratory pigments
The respiratory pigment hemoglobin has evolved independently many times and is by far the most widespread respiratory pigment in animals. In occurs in almost all vertebrates. It also occurs in annelid worms, such as earthworms, and in some members of at least seven other phyla.
Hemoglobin is an iron-containing protein to which O2 binds at the molecular sites (called heme sites) where iron atoms occur. In mammals and most other vertebrates, each hemoglobin molecule is an assembly of four protein subunits, each of which contains one iron atom (in heme; see Chapter 3). In all vertebrates, hemoglobin is found inside red blood cells, where it is responsible for their red color.
Fully oxygenated hemoglobin is bright crimson red. Fully deoxygenated hemoglobin, however, is purple-red. The finger probes (pulse oximeters) that hospital patients wear to track blood oxygenation work by detecting how much hemoglobin is one color and how much is the other.
Red blood cells turn over rapidly, especially in mammals. In humans, each red cell circulates in the blood for only about 120 days before it is broken down and replaced by a new cell synthesized in the bone marrow. Under normal conditions, your blood contains about 5 billion red cells per milliliter, and your bone marrow produces about 2 million new cells every second.
Hemocyanin is the second most important respiratory pigment. It is found widely in mollusks, including squid, and in arthropods such as crabs, lobsters, horseshoe crabs, and spiders. The molluscan and arthropod forms evolved independently. Like hemoglobin, hemocyanin is a metal-containing protein to which O2 binds at the molecular sites where metal atoms occur. The metal, however, is copper rather than iron. Hemocyanin is never found inside blood cells. Instead, it is always simply dissolved in the blood plasma.
Oxygenated hemocyanin is blue, and deoxygenated hemocyanin is colorless. Hemocyanin is usually not concentrated enough in the blood to give the blood a strong color, but in some animals that have lots of hemocyanin, the blood leaving the gills is a bright cobalt blue!
Respiratory pigments combine with O₂ reversibly
What gives respiratory pigments their ability to transport O2? Their most important functional characteristic is that they combine reversibly with O2. They take up O2 when O2 is abundant in their surroundings. They release O2 when their surroundings are O2-depleted. Both the uptake and release of O2 occur automatically, and very quickly, as a simple consequence of the chemical properties of the pigments.
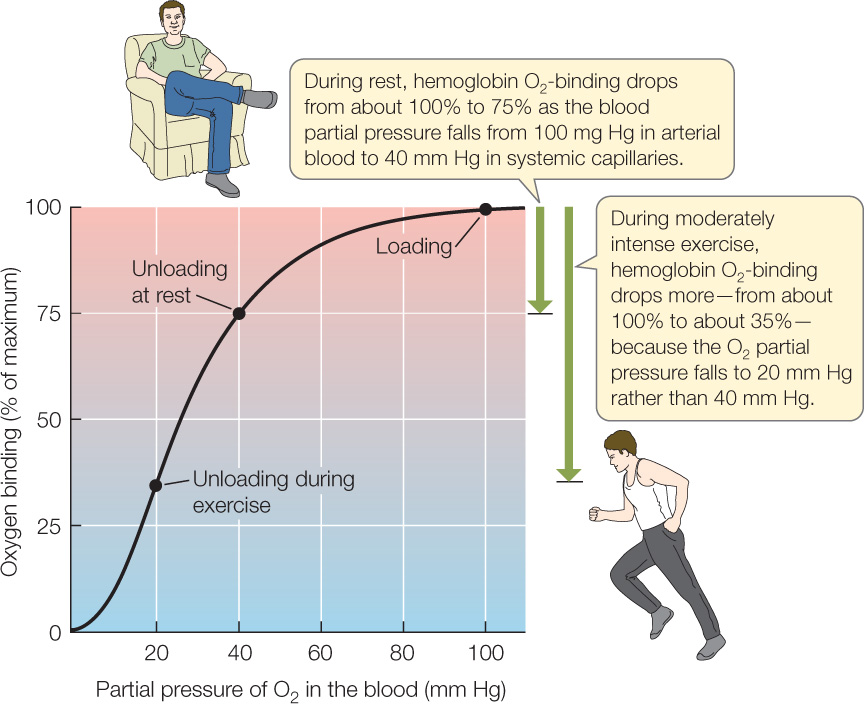
This chemical behavior is summarized with a graph like that shown in FIGURE 32.20. Such a graph is called an oxygen equilibrium curve, and the particular curve shown here is typical for adult humans. The x axis shows the partial pressure of O2 in the blood (this is a measure of the concentration of O2 dissolved in the blood plasma). The hemoglobin in the blood has a maximum amount of O2 with which it can combine. The y axis shows the amount of O2 bound to hemoglobin at each partial pressure as a percentage of this maximum.
Go to ACTIVITY 32.4 Oxygen-Binding Curves
PoL2e.com/ac32.4

Go to ANIMATED TUTORIAL 32.2 Hemoglobin Loading and Unloading Simulation
PoL2e.com/at32.2
Let’s focus on blood O2 transport in a person. The O2 partial pressure in the alveoli of a person’s lungs is kept near 100 mm Hg because of the control of breathing we discussed in Concept 31.3. This means that the O2 partial pressure in blood oxygenated in the alveoli is also kept near 100 mm Hg. As shown by the “Loading” point on the graph in Figure 32.20, human hemoglobin combines with a maximum amount of O2—about 100 percent of the O2 it can possibly carry—when the O2 partial pressure is near 100 mm Hg. In short, human hemoglobin becomes highly oxygenated in the lungs.
678
In the systemic tissues of a person at rest, the O2 partial pressure, on average, is about 40 mm Hg. The “Unloading at rest” point on the graph shows the consequences. O2 binding is only about 75 percent of maximum when human hemoglobin is at 40 mm Hg. When highly oxygenated blood from the lungs first flows into the capillaries of the systemic tissues, its oxygen binding is about 100 percent, but as soon as it is exposed to the partial pressure of 40 mm Hg that prevails in the systemic tissues, it releases some O2 so that its oxygen binding falls to 75 percent. This is the process by which hemoglobin releases O2 to the tissues. Later, when the blood flows back into the lungs, the hemoglobin is exposed once more to a partial pressure of about 100 mm Hg and therefore takes up O2, so that its oxygen binding is once again about 100 percent.
During exercise, hemoglobin can greatly increase the amount of O2 it releases in the exercising muscles. The O2 partial pressure in exercising muscles often falls to about 20 mm Hg during moderately intense exercise because the muscle mitochondria are using O2 rapidly. When hemoglobin is exposed to this partial pressure, its oxygen binding falls to only 35 percent (see “Unloading during exercise” in Figure 32.20). The hemoglobin thus releases much more of its O2 than it does in resting tissues.
In summary, there are two major ways that the circulatory system increases O2 delivery when we exercise. First, we circulate blood more rapidly than at rest (by increasing heart rate and stroke volume). Second, our hemoglobin releases more than twice as much O2 each time it flows from the lungs to the exercising muscles.
CHECKpoint CONCEPT 32.5
- By what factor does hemoglobin increase the capacity of human blood to carry O2?
- What do we mean when we say that hemoglobin and hemocyanin combine reversibly with O2?
- Myoglobin, the red pigment inside muscle cells that are specialized for endurance exercise (see Concept 33.3)—giving the cells a red color—is actually a type of hemoglobin. There’s a property of respiratory pigments called oxygen affinity. It is a measure of how strongly a respiratory pigment and O2 are chemically attracted. Red muscles need O2 because they make ATP aerobically. Keeping this in mind, would you expect myoglobin to have a higher or lower oxygen affinity than the hemoglobin in the blood? Explain.
Question 32.2
How can the arrangement of blood vessels help warm the swimming muscles in warm-bodied fish such as tuna?
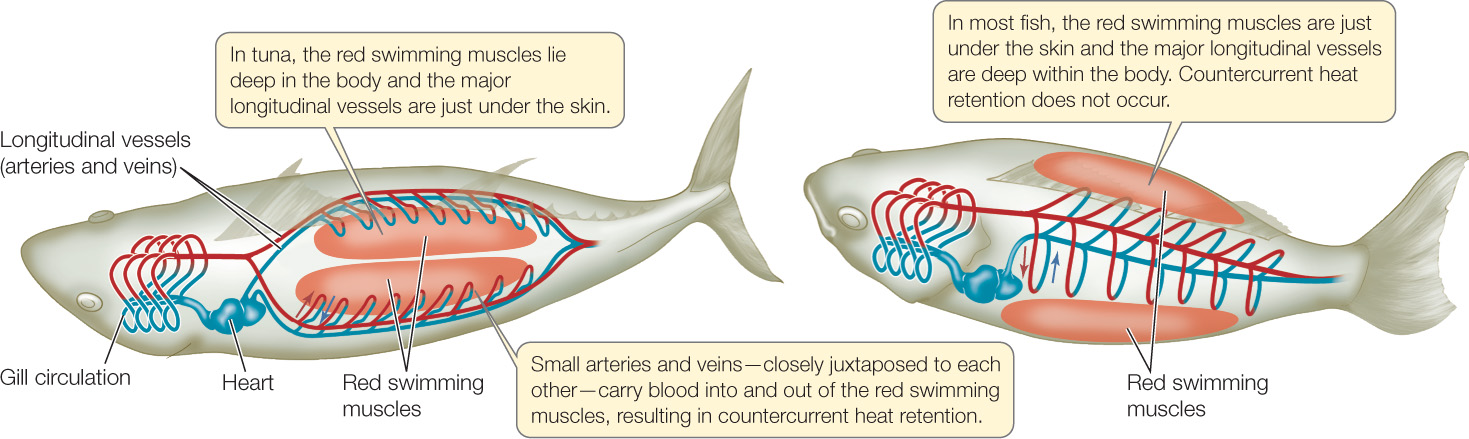
ANSWER The red swimming muscles produce considerable heat from their metabolism during steady, long-duration swimming in high-performance species of fish. In most species, however, this heat is lost to the environmental water as fast as it is produced, and the swimming muscles are not warmed by the heat they produce. This is true regardless of body size because heat moves readily through water, and in the gills it merely needs to move from one watery fluid, the blood, to another, the environmental water, to escape from the fish.
The key to keeping heat in the swimming muscles, so that they are warmed, is to prevent the heat they produce from leaving them. This is not possible in most fish because of the arrangement of the arteries and veins in the vasculature of the swimming muscles (FIGURE 32.21). Tuna (and certain sharks, such as great whites) have evolved a unique arrangement of arteries and veins to carry blood to and from their red swimming muscles, which are deep in the body (see Figures 32.17 and 33.14). In this arrangement, as veins leave the swimming muscles—carrying heat with them—they come into close contact with the arteries carrying blood into the muscles. This arrangement allows countercurrent heat exchange to take place. Heat moves out of the venous blood into the arterial blood, and the arterial blood carries the heat back into the swimming muscles. Heat made by the muscles thus has a hard time exiting them (very unlike the case in most fish), and the muscles are warmed to temperatures higher than that of the environmental water.
679