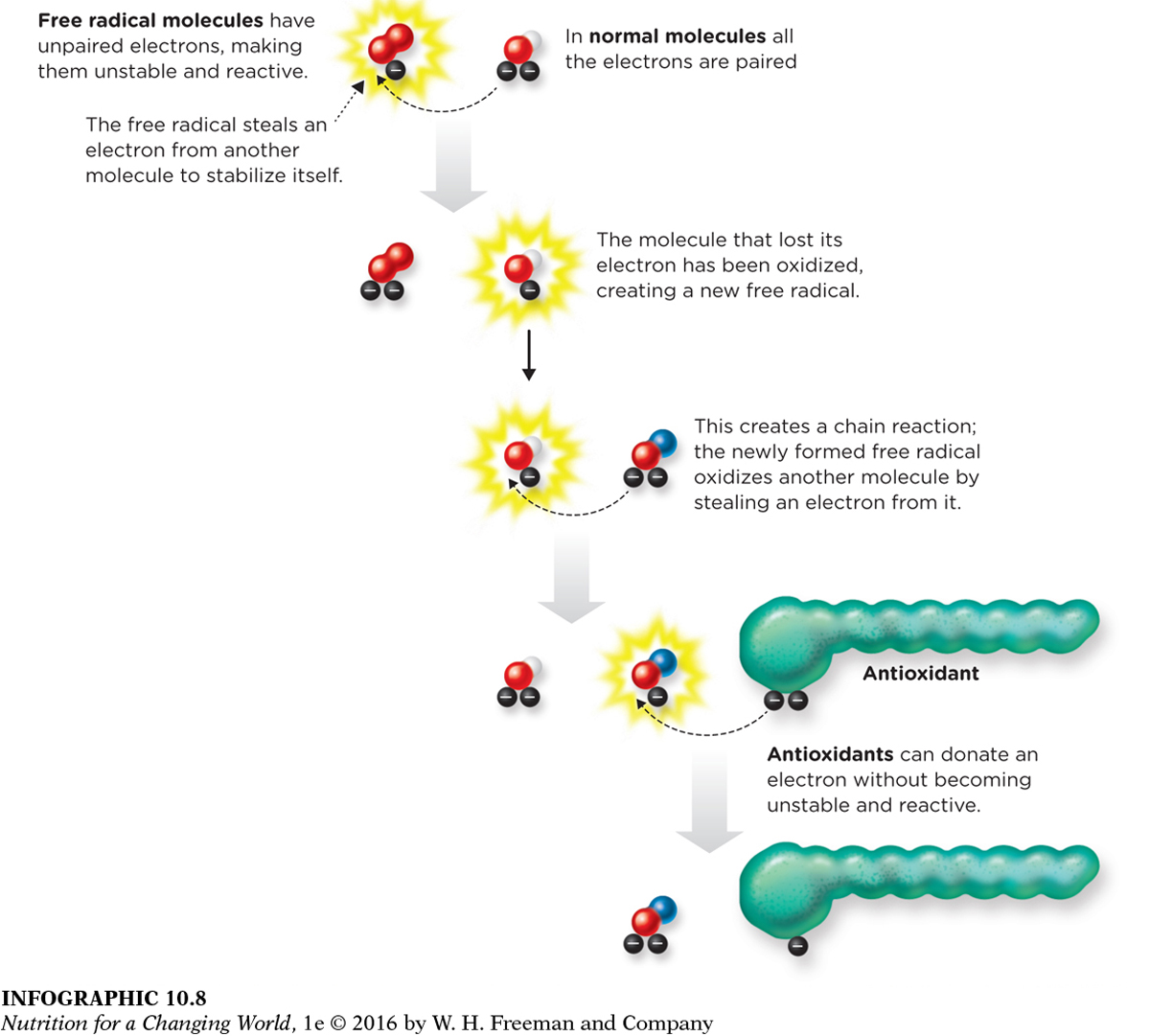
INFOGRAPHIC 10.8 Antioxidants Defend Against Oxidative Damage Caused by Free Radicals Free radicals are molecules containing unpaired electrons, which makes them highly reactive. The free radical either causes oxidative damage by reacting with another molecule and chemically modifying it, or it stabilizes itself by stealing an electron from a nearby molecule, which creates a new free radical and begins a chain reaction. Antioxidants are able to stop the chain reaction, by donating an electron.