Chapter Introduction
MET-
METABOLISM: UNDERSTANDING THE INTERACTIONS AND TRANSFORMATIONS IN LIVING CELLS
UNDERSTANDING METABOLISM
KEY IDEAS
Metabolism is all the life-
sustaining chemical reactions that occur in living organisms that convert one molecule into another molecule. Many metabolic processes fall into one of two broad categories, catabolism and anabolism. Catabolism is the breakdown of large molecules into smaller ones, and is generally accompanied by the release of energy.
Anabolism is the synthesis of large molecules from smaller ones, requiring an input of energy that is obtained from catabolic reactions.
Since digestion occurs within the lumen of the gastrointestinal tract, which is fundamentally still outside the body, digestion is not considered to be metabolism.
WHAT IS METABOLISM?
Metabolism is all the life-
The molecule that an enzyme acts on is referred to as a substrate, and the modified molecule that the reaction yields is called the product. Many enzymes require small, organic, nonprotein molecules called coenzymes to function. Coenzymes bind at a location on the enzyme known as the active site and form an active enzyme, which is only then capable of catalyzing its designated reaction. Vitamins C and K, and all of the B vitamins function as coenzymes.
How Do Enzymes Work?
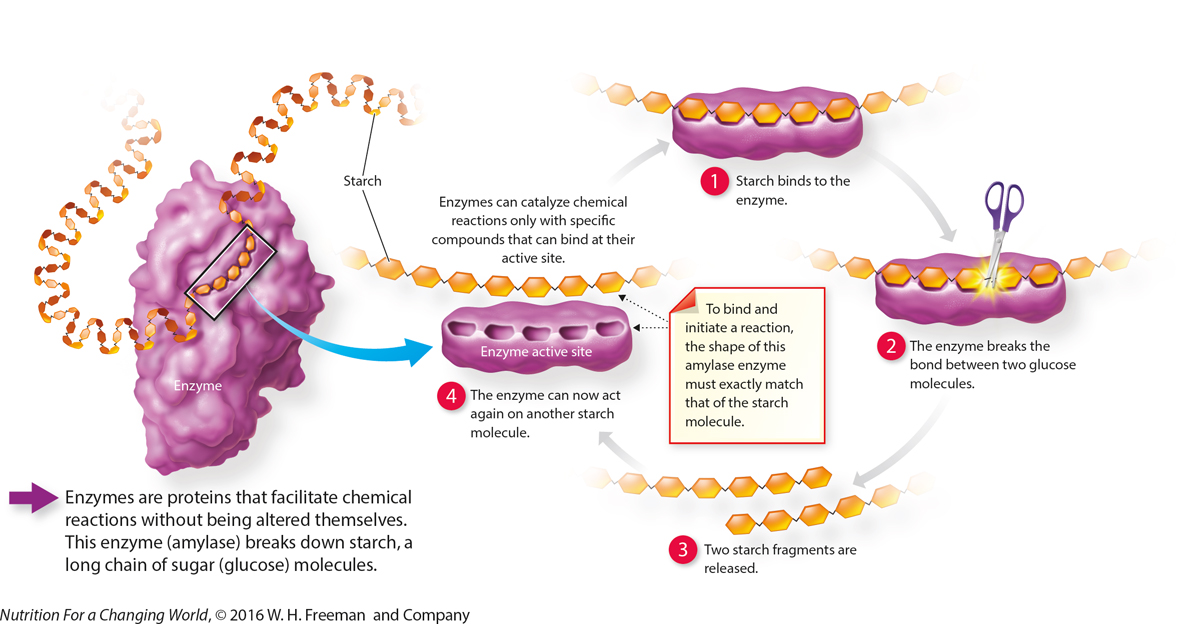
|
|
QuestionWhy won’t this enzyme break down lipids or proteins? Enzymes can only catalyze chemical reactions with the specific compounds that can bind at their active site. The enzyme amylase breaks down carbohydrates in the form of starch and is not compatible with lipids or proteins. |
How Do Coenzymes Work? Coenzymes associate with enzymes to form an active complex that is capable of catalyzing a chemical reaction. Some vitamins function as coenzymes.
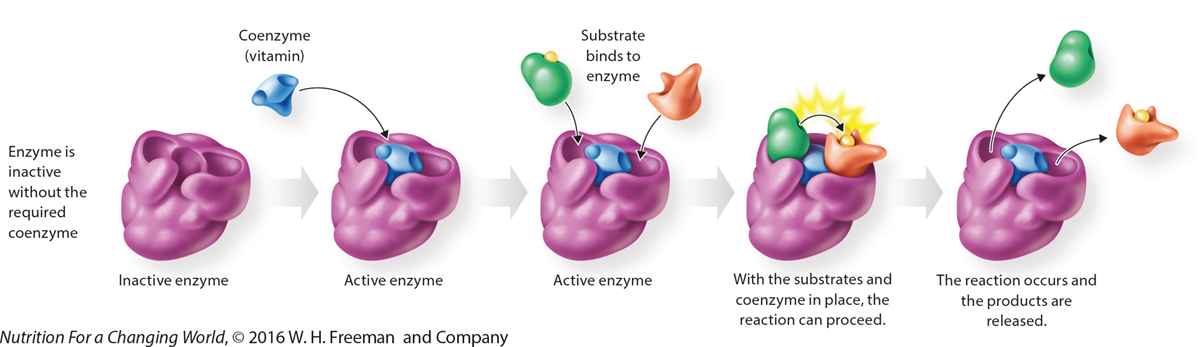
|
|
QuestionDescribe the basic function of a coenzyme. Coenzymes are small, organic, molecules that are not proteins. They bind at a location on an enzyme known as the active site. Only after binding to the active site is the enzyme active, and capable of catalyzing a reaction. |
Many of the chemical transformations that occur within cells require multiple individual reactions to be completed in a series. For this reason, cellular metabolism is typically organized into metabolic pathways. Each pathway transforms its original substrate into a final product or products through a sequence of linked enzyme-
Many metabolic processes fall into one of two broad categories: anabolism and catabolism. Anabolism is the synthesis of large molecules from smaller ones, requiring an input of energy. Common anabolic processes are those that synthesize proteins from amino acids, glycogen from glucose, and triglycerides from sources of excess calories (such as glucose and amino acids). Cell division and growth are also anabolic processes.
Catabolism, on the other hand, is the breakdown of large molecules into smaller ones, and is generally accompanied by the release of energy. Catabolic processes supply the fuels that are needed to drive anabolism, and they can also provide the substrates needed for a number of anabolic processes. The balance between all anabolic and catabolic processes over the course of several days will determine if an individual’s weight will remain stable, or whether he or she will experience a change in body weight. For example, if the total number of catabolic processes exceeds that of anabolic processes, an individual’s body weight would decrease, as adipose tissue and muscle mass are lost.
Energy metabolism is the chemical reactions that are involved in storing fuels, or breaking them down to provide the energy necessary to drive a variety of chemical reactions and other cellular processes (such as active transport and muscle contractions). This energy comes from one of two main fuel sources: glucose and fatty acids. Both of these fuels are rich in chemical energy, stored in the chemical bonds that hold each molecule together. As fuels are slowly metabolized and broken down, energy is released and the product of each reaction contains less energy than the starting substrate. This released energy is not in a form the body cells can use; it must be converted into a molecule called adenosine triphosphate (ATP).
Commonly referred to as the cell’s energy currency, ATP stores chemical energy in the bonds of its three phosphate groups. When our cells need energy, they typically break the bond between the last two phosphates, releasing the stored energy and forming adenosine diphosphate (ADP). (The “di” in diphosphate means “two,” as in two phosphates; the “tri” in “triphosphate” refers to its three phosphates.)
MET-
Adenosine Triphosphate (ATP) is produced during energy metabolism. ATP has a high energy content and is often referred to as the energy currency of cells.
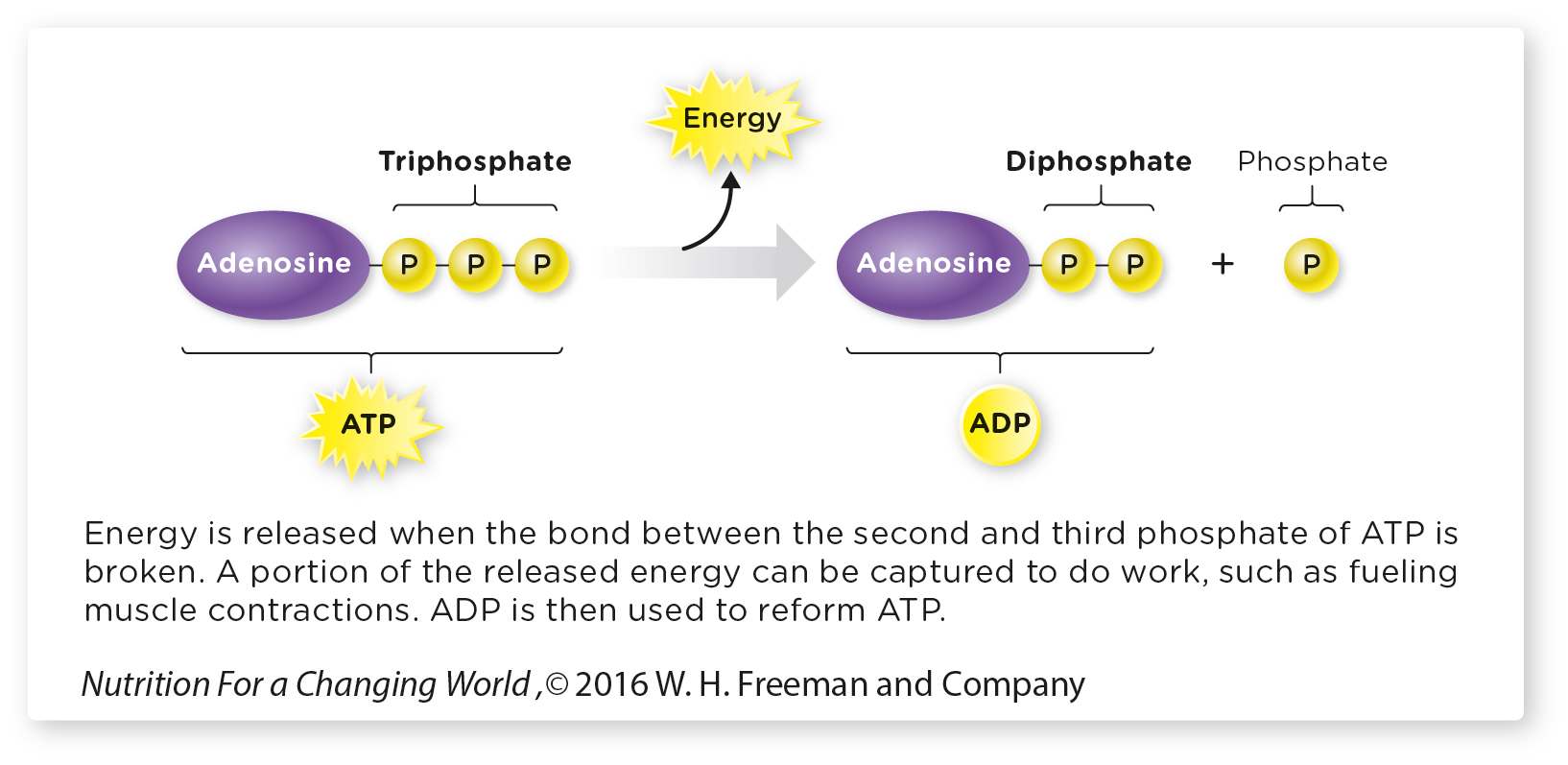
You can think of the energy in glucose and fat as the value of a gold brick: It’s worth a lot of money, but you can’t buy a cup of coffee with it. ATP, however, is like bills and coins—
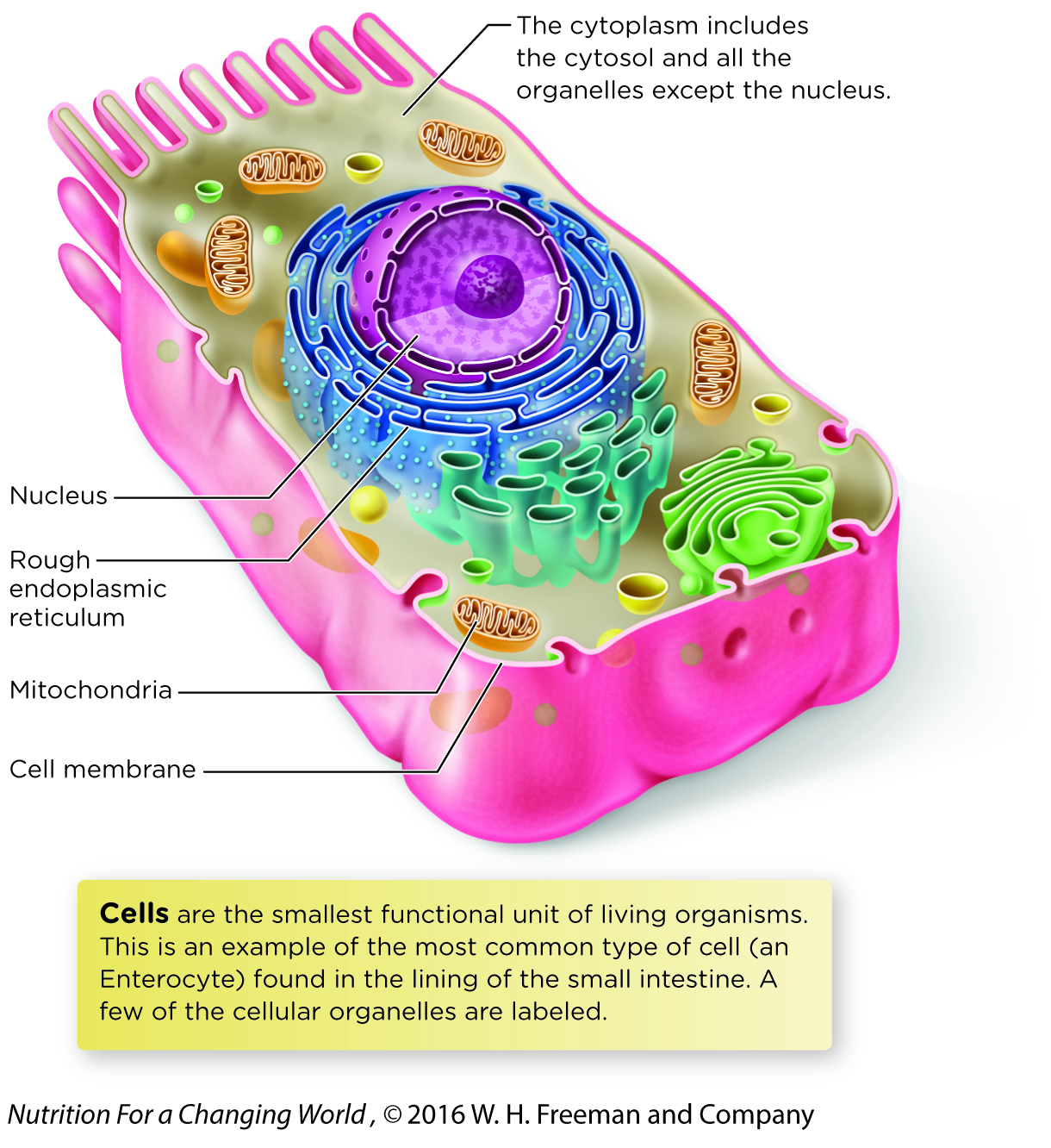
The reactions of energy metabolism primarily occur in two cellular compartments, the cytosol and the mitochondria. Recall that cells are surrounded by a cell membrane. Within the cell membrane is an aqueous fluid called the cytosol, as well as a number of cellular organelles and other structures. The membrane-
All other pathways involved in the production of ATP occur in mitochondria, which produce the majority of ATP in most cells. Mitochondria are organelles surrounded by a double-
MET-
Oxidation-
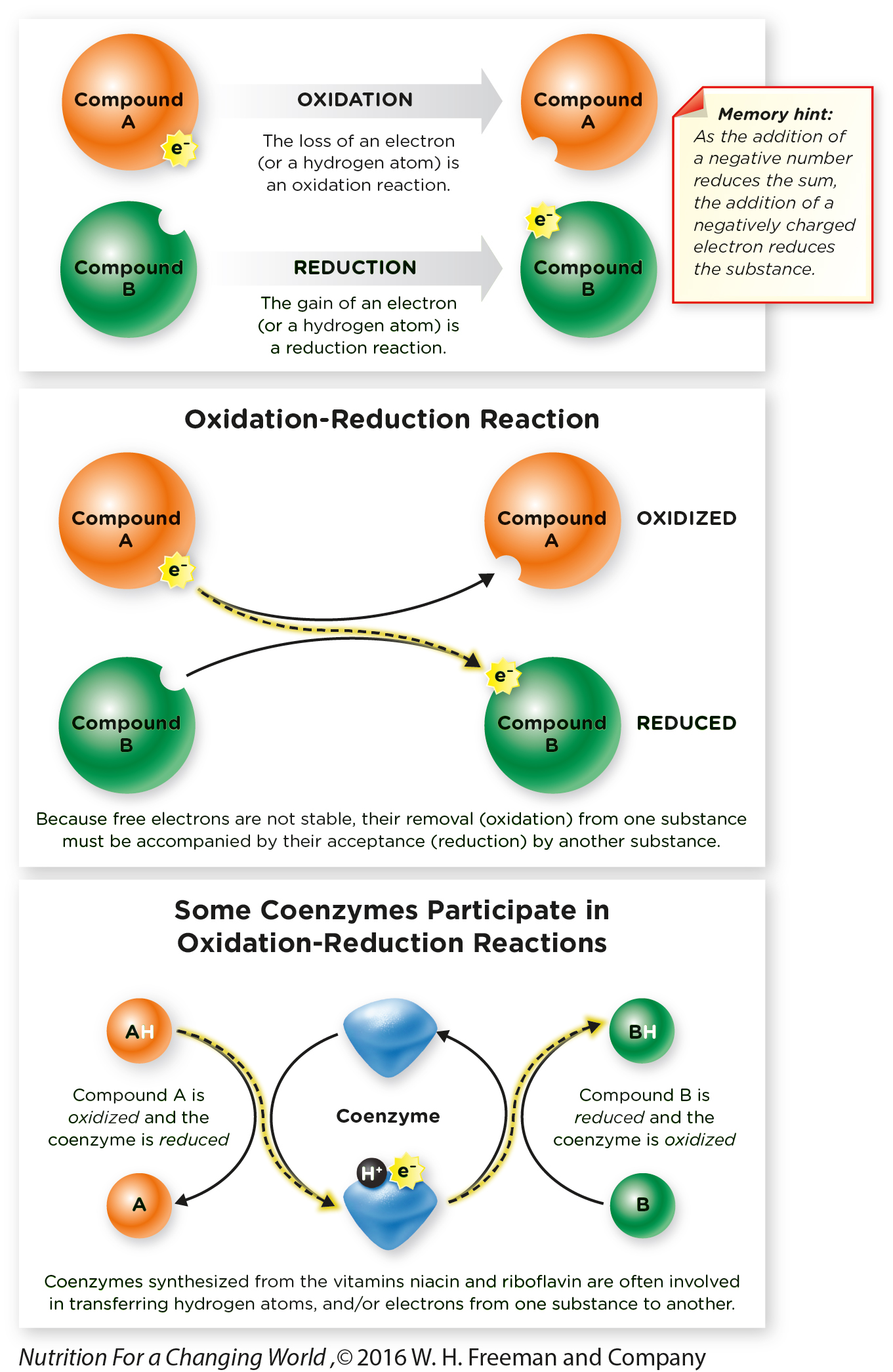
What Are Oxidation-
