Communities can have distinct or gradual boundaries
If we are to understand ecological communities, we first need to understand how communities change across the landscape and how they are categorized. As we will see, because many species move between communities, drawing boundaries around a community can be difficult. For example, many birds migrate every spring and fall, and amphibians spend their larval life in aquatic communities and their adult life in terrestrial communities. In this section, we will discuss community boundaries and investigate how communities are affected when community boundaries are either distinct or gradual.
Community Zonation
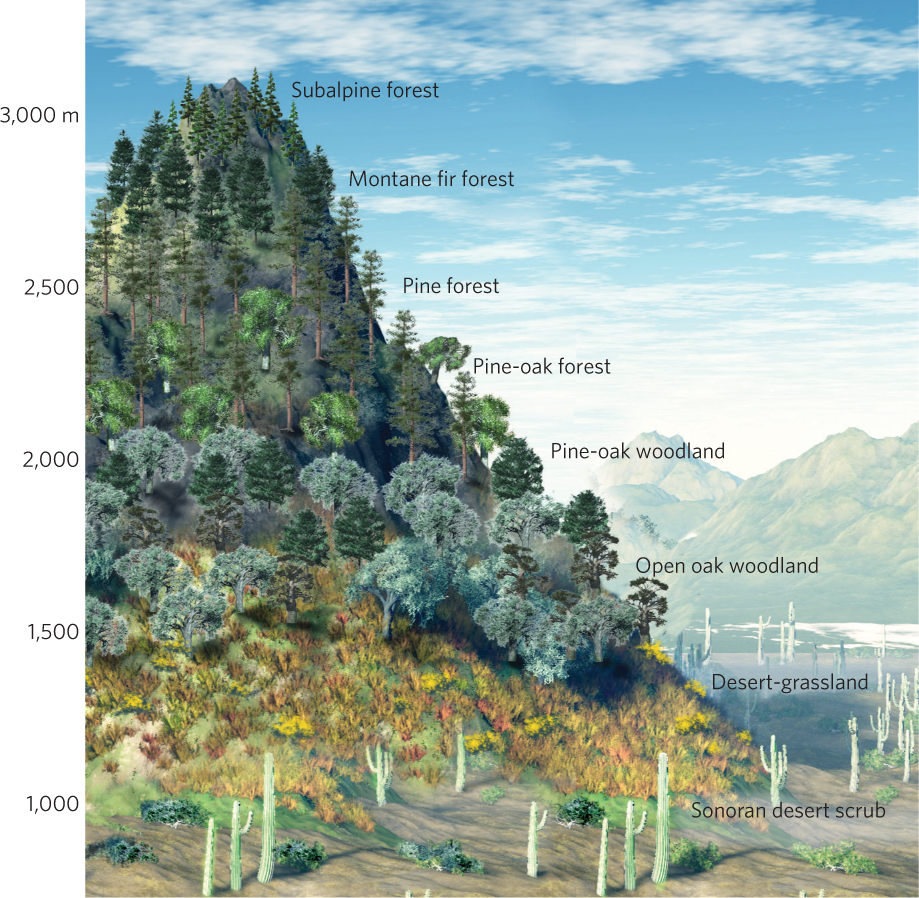
One of the most noticeable features of communities is that the species composition of the community changes as one moves across the landscape. With the change in environmental conditions, some species become better able to survive and compete. For example, if you were to walk from the base to the top of a mountain in the Santa Catalina Mountains in Arizona, you would observe striking changes in the vegetation, as illustrated in Figure 18.1. At the base of the mountains, you would first encounter desert-adapted plants including rabbit bush (Franseria deltoidea) and creosote bush. As you begin ascending the mountain, you would find grasses, some shrubs such as narrowleaf goldenbush (Haplopappus laricifolius), and a few scattered oaks. As you approach the top of the mountain, you would reach a forest of ponderosa pines and southwestern white pine (Pinus strobiformis), followed by a forest containing Engelmann spruce (Picea engelmanni), white fir (Abies concolor), and subalpine fir. The zones in which each species flourishes reflect different tolerance ranges for temperature and moisture availability, as well as different abilities to compete with other species. There are similar changes in the animal species that live at different elevations on the mountains. The changes in plants and animals at different elevations create continuous changes in community composition from the base of the mountains to the highest peaks.
415
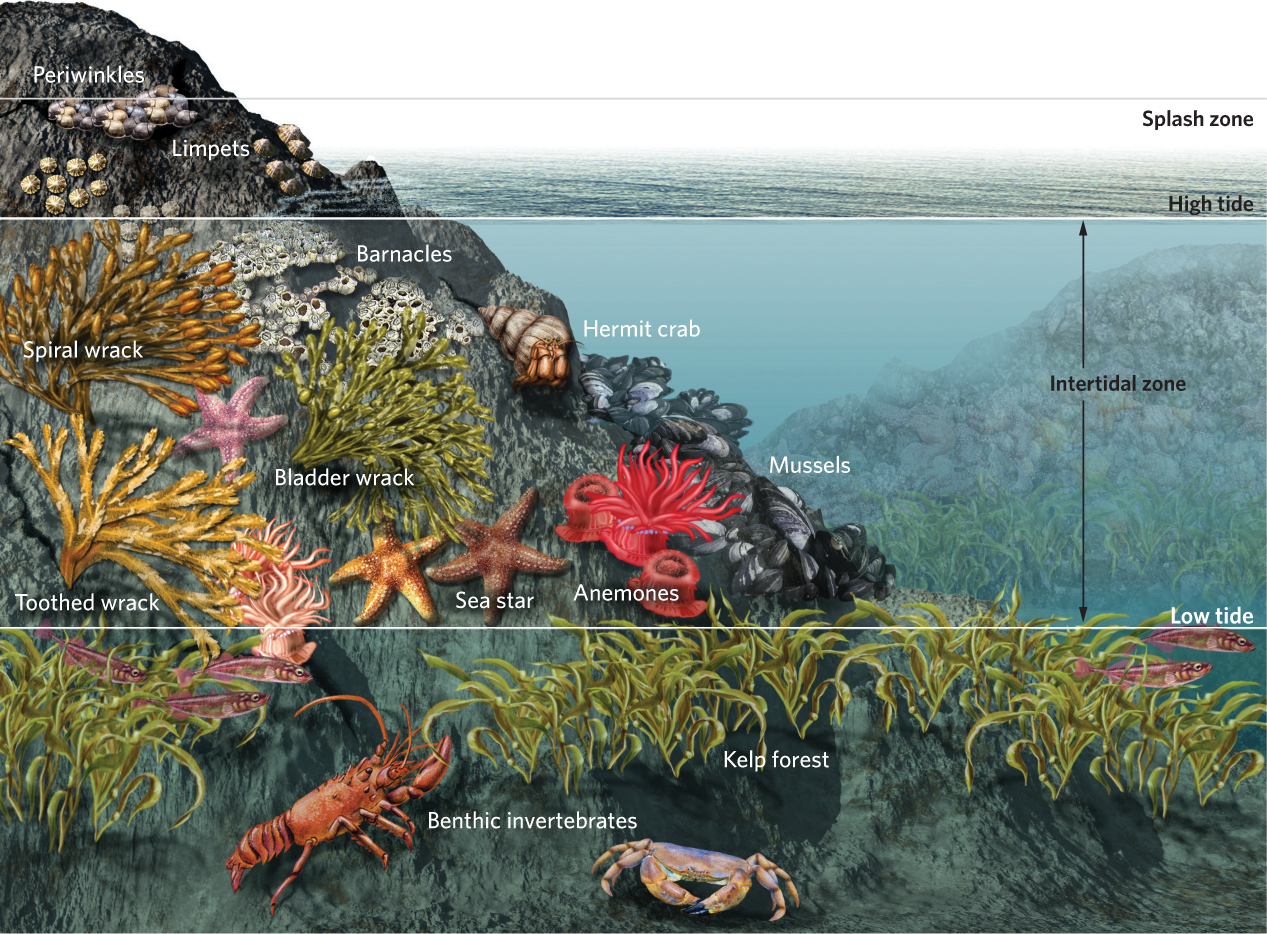
Zonation also occurs in aquatic communities, as depicted in Figure 18.2. We saw an example of this in Chapter 16 when we discussed competition between species of barnacles on rocky coasts of Great Britain. Due to a combination of competition and resistance to desiccation, stellate barnacles live in the upper intertidal zone whereas the rock barnacles live in the lower intertidal zone. Occupation of different zones is a pattern that is repeated among a wide variety of species in intertidal communities. For example, we find kelp forests below the intertidal zone, and a variety of seaweeds, mussels, anemones, rock barnacles, and hermit crabs occupy the lower and middle intertidal zones. In the upper intertidal zone we find limpets, which are a type of gastropod, and stellate barnacles. Higher up on the shore an area known as the splash zone is home to more limpets and a type of snail known as the periwinkle (Littorina littorea). As we saw in the case of the two barnacle species, the distribution of species in the different zones of the coastline reflects a combination of tolerance to changing abiotic conditions and the outcome of species interactions that include competition, predation, and herbivory.
416
Categorizing Communities
Ecologists generally categorize communities as they do biomes—either by their dominant organisms or by physical conditions that affect the distribution of species. In North America, for example, we might examine a beech-maple forest community in Ontario, a pine savanna community in Virginia, or a sagebrush community in Wyoming. Each of these communities is named for the dominant plants that are present. In aquatic systems, we might focus on physical characteristics—such as a stream community, lake community, or wetland community—or on the dominant group of organisms in that system, such as a coral reef community.
When ecologists study communities, they rarely study every species in the community, which could number in the hundreds or thousands, but rather focus on a subset of species that live in an area. For example, researchers studying a wetland community might study only bacteria, algae, snails, crustaceans, insects, amphibians, or fish. They might also examine several of these groups simultaneously.
Ecotones
Ecotone A boundary created by sharp changes in environmental conditions over a relatively short distance, accompanied by a major change in the composition of species.
When we try to categorize communities, it is clear that some communities have distinct boundaries that are either natural or constructed by humans. For example, the natural boundary between a lake community and a forest community can be clearly drawn at the water’s edge. This boundary exists because there is a sharp change in the environmental conditions as we leave one community and enter the adjacent community. Similar sharp changes in environmental conditions occur, for example, when there is an abrupt change in the type of soil due to the underlying geology of an area or when an individual moves from north-facing to south-facing slopes of mountains that have large differences in temperatures and moisture. Humans can also create distinct community boundaries. For example, an area of forest that has been cleared for agriculture creates a clear boundary between the field community and the forest community. Sharp changes in environmental conditions over a relatively short distance, accompanied by a major change in the composition of species, creates a boundary known as an ecotone (Figure 18.3). Although some species move between the adjacent communities that come together to form the ecotone, most species live in one of the communities and spread into the ecotone. As a result, ecotones typically support a large number of species, including those from the adjoining habitats and species adapted to the ecotone’s special conditions.

417
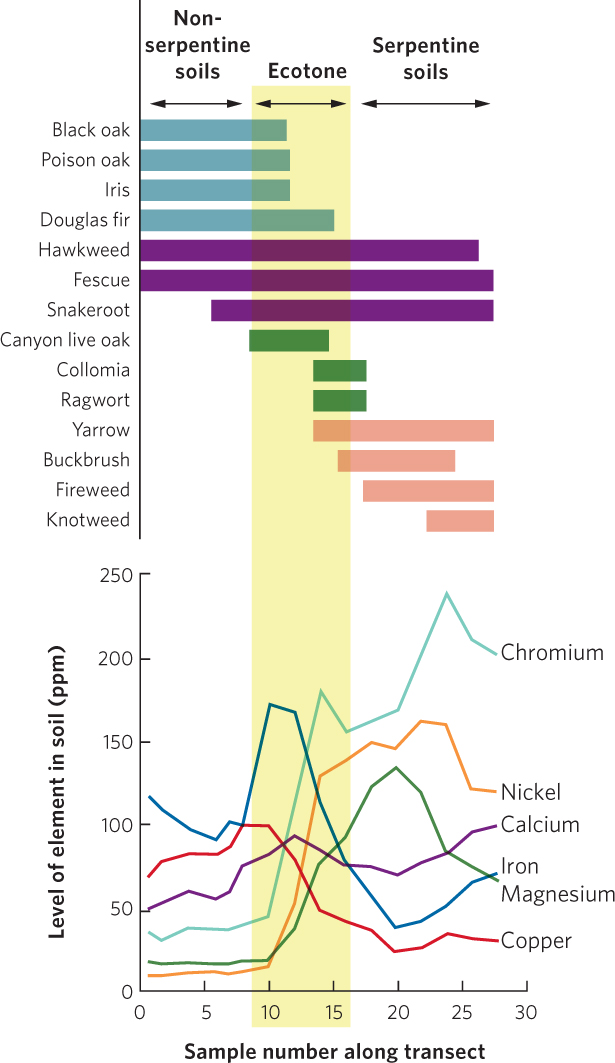
One way to document the existence of an ecotone is to use a line-transect survey to determine the abundances of different species along an environmental gradient. When an ecotone is present, we expect to observe sharp changes in the distributions of species as we leave one community and enter the adjacent community. An example can be found in plant communities on serpentine soils. These soils are derived from underlying rock that contains heavy metals, such as nickel, chromium, and magnesium, that are toxic to many plants. Serpentine rock outcrops exist in small patches across the landscape in many different parts of the world. Serpentine soils are typically low in nutrients such as nitrogen and phosphorus. Because of the harsh conditions, most plant species cannot live in serpentine soils, although some species of plants have evolved an ability to tolerate them (Figure 18.4).

In one study in southern Oregon, researchers conducted a line-transect survey that stretched from nonserpentine soils to serpentine soils. They measured the concentrations of several metals and quantified the presence of numerous plant species. Concentrations of chromium, nickel, and magnesium all increased in the shift from nonserpentine soils to serpentine soils, as you can see in the bottom half of Figure 18.5. When they examined the distribution of the plants, as shown in the top half of the figure, they found that species such as black oak (Quercus kelloggii) and poison oak (Rhus diversiloba) did not grow on the serpentine soils, whereas canyon live oak (Quercus chrysolepis) and ragwort (Senecio integerrimus) were found almost entirely in the ecotone where the two soils come together, and species such as fireweed (Epilobium minutum) and knotweed (Polygonum douglasii) were found only in serpentine soils. These species show distinct boundaries as one moves across the gradient of nonserpentine to serpentine soils. As you can also see in the transect data, a few species such as hawkweed (Hieracium albiflorum) and fescue (Festuca californica) were found across the entire gradient. From these data we can conclude that while some species are largely restricted to either serpentine or nonserpentine soils, the highest number of species occurs within the ecotone.
418
Communities With Interdependent Versus Independent Species Distributions
As we have noted, a community is often described by the dominant species that live within it. Although we know that species interact with each other, for many years ecologists questioned whether species in a community were found together because they depended on each other or because they had similar habitat needs.
Interdependent communities Communities in which species depend on each other to exist.
Interdependent communities are those in which species depend on each other to exist. In the case of the social spider, for example, the various species of spiders determine the growth and reproduction of all spiders and determine the life span of the community. In the early twentieth century, plant ecologist Frederic Clements proposed that most communities function as interdependent communities and that they act as superorganisms. He compared individual species to the different parts of an organism’s body, all of which require each other for survival.
Independent communities Communities in which species do not depend on each other to exist.
Independent communities are those in which the species do not depend on each other to exist. Independent communities are composed of species that live in the same place because they have similar adaptations and nutrient requirements. Although each species has a somewhat different range of conditions under which it can live, the community that we see in a given location reflects the overlapping ranges of the species that exist within it. In other words, similar habitat requirements happen to put various species in the same place at the same time. Plant ecologist Henry Gleason rejected Clements’s superorganism metaphor and proposed that most communities consist of species with independent distributions.
419
Differentiating Between Interdependent and Independent Species Distributions
How can we determine whether a community is comprised of an interdependent or an independent group of species? One approach has been the use of line-transect studies. If species distributions are independent, we should be able to observe gradual changes in species composition as we move along a line transect. Each species will appear and disappear at different points along the line because of the unique habitat requirements of each species.
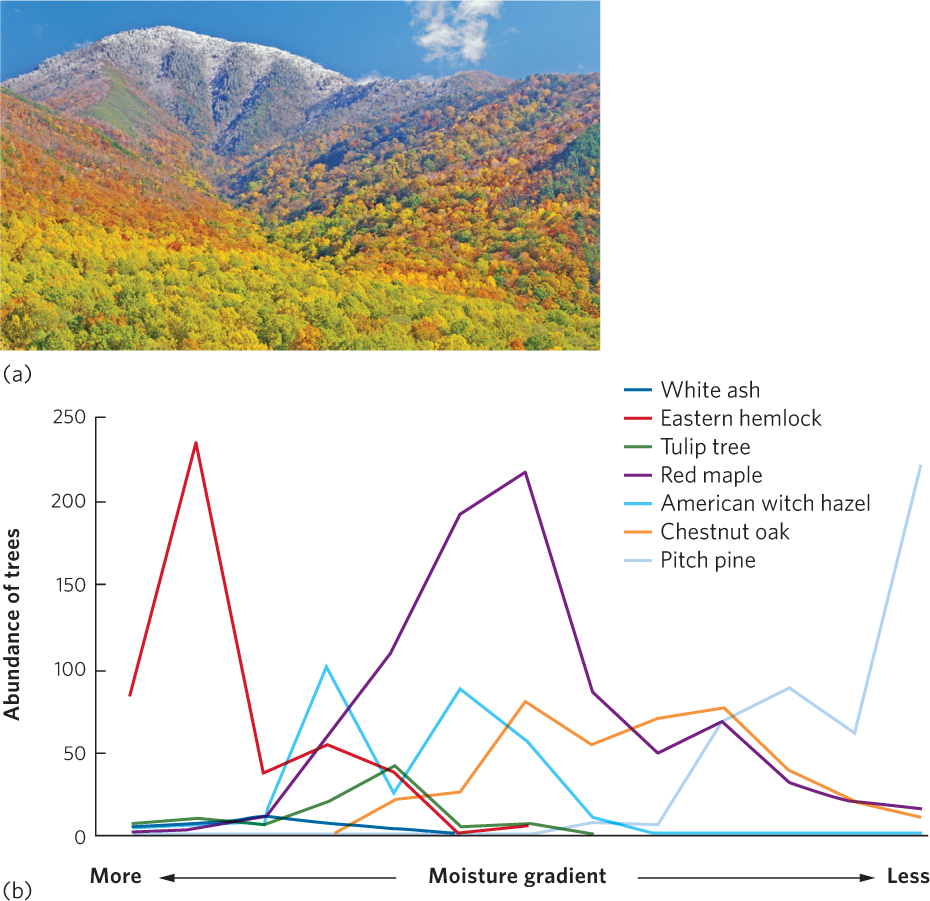
A classic study using this approach was conducted during the 1950s by Robert Whittaker who surveyed the distributions of plant species in the Great Smoky Mountains on the border between Tennessee and North Carolina (Figure 18.6a). At different elevations that varied in temperature and moisture, he calculated the number of stems present for each plant species. As you can see in Figure 18.6b, different species appeared and disappeared at different points along the moisture gradient, and each species achieved its peak abundance at different points. This study and other studies by Whittaker provided strong evidence that when environmental conditions change gradually along a gradient, species in the community commonly show gradual changes in abundance that are independent of each other.
Observing species distributions along environmental gradients is one way to test the independence of species distributions. However, even when we see abrupt changes in species distributions we cannot be sure whether species in a community are independent or interdependent. An abrupt transition may reflect an abrupt change in abiotic conditions where many species simply cannot exist under the changed conditions. For example, fish and other aquatic organisms are not restricted to live in a lake because they are necessarily interdependent; they are restricted to the lake because they lack the adaptations to live on land. To determine whether species are interdependent and therefore rely on each other for their existence, we need to examine how well species perform when other species are removed. If species rely on each other to persist, then removing a species from a community should cause other species in the community to decline. If they don’t need each other to persist, then removing a species should leave other species in the community unaffected or even improve their fitness if they happen to compete with the species that is removed.
420
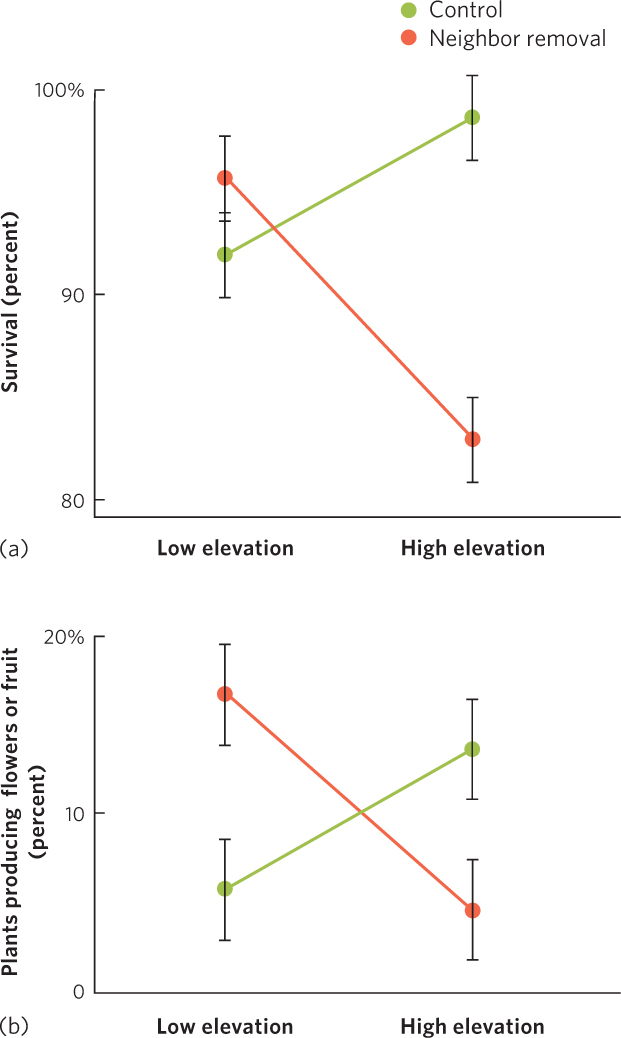
Recent studies have increasingly found that some species do rely on each other to persist, particularly in communities that experience harsh environmental conditions, such as extreme high or low temperatures or low moisture. For example, in a large experiment conducted in 11 locations around the world, researchers examined how 115 plant species growing at either low or high elevation in alpine tundra biomes responded when a neighboring species was removed. The researchers then measured the percentage of plants that survived as well as the percentage of plants that produced flowers. Removing neighboring plants at low elevation sites caused an increase in the survival of the remaining plants, as shown in Figure 18.7a. This also caused an increase in the percentage of plants that produced flowers or fruit, which you can see in Figure 18.7b. In contrast, removing neighboring plants at high elevation sites caused a decrease both in the survival of the remaining plants and in the percentage of plants that produced flowers. Plants living under the harsher conditions of high elevations were helped by the presence of neighboring species because those species reduced the harsh winds, provided shade, or offered protection from herbivores. Such experiments have taught ecologists that while most communities appear to be composed of species with independent distributions, species living under harsh environmental conditions frequently depend on other species in the community.