Communities are organized into food webs
Food chain A linear representation of how different species in a community feed on each other.
Even the simplest communities are composed of a large number of species. To understand the relationships among the species, ecologists have found it helpful to categorize species in a community into food chains and food webs. Food chains are linear representations of how species in a community consume each other and therefore how they transfer energy and nutrients from one group to another in an ecosystem. Food chains greatly simplify species interactions in a community. In contrast, food webs are complex and realistic representations of how species feed on each other in a community and include links among many species of producers, consumers, detritivores, scavengers, and decomposers. In constructing food webs, ecologists draw arrows that indicate consumption—and therefore the movement of energy and nutrients— from one group to another. You can see an example of a food web in Figure 18.18.
Food web A complex and realistic representation of how species feed on each other in a community.
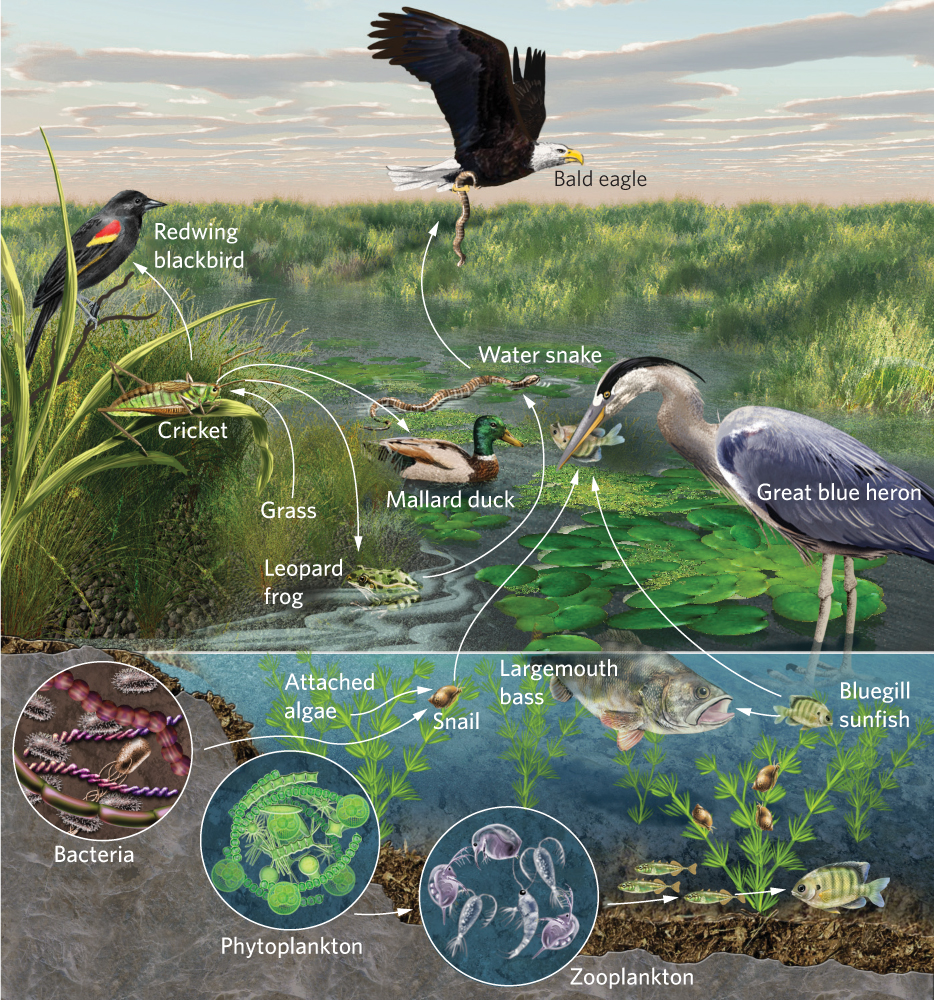
Food webs help us understand the feeding relationships of ecological communities, which is important because feeding relationships help determine whether a species can exist in a community and whether it will be rare or abundant. The species richness in a food web can often be quite high, which makes it challenging to comprehend how the abundance of one species is affected by other species in the community. To simplify this challenge, ecologists categorize species into trophic levels.
Trophic Levels
Trophic level A level in a food chain or food web of an ecosystem.
You may recall the concepts of producer and consumer from our overview of basic ecological concepts in Chapter 1. These represent broad categories of trophic levels, which are the levels in a food chain or food web of an ecosystem. All organisms at a particular trophic level obtain their energy in a similar way. Producers are the autotrophs, including algae such as phytoplankton, and plants that convert light energy and CO2 into carbohydrates through photosynthesis. Producers form the first trophic level of a food web. Consumers can be subdivided into primary consumers, secondary consumers, and tertiary consumers. Primary consumers are those species that eat producers. In the aquatic food web portrayed in Figure 18.18, the primary consumers include zooplankton that eat algae and snails that eat plants. Secondary consumers are those species that eat primary consumers. In our lake food web these secondary consumers include small fish that eat the zooplankton and ducks that eat the snails. Some communities support tertiary consumers, which eat secondary consumers; for example, large fish in a lake are tertiary consumers because they consume small fish. In addition to these producers and consumers, food webs also include consumers of dead organic matter such as scavengers, detritivores, and decomposers. One of the challenges in thinking about placing species into specific trophic levels is that many species are omnivores, meaning that they can feed at several trophic levels. For example, crayfish consume algae, which would make them primary consumers, but they also feed on insects and detritus, which would make them secondary consumers and detritivores.
Primary consumer A species that eats producers.
Secondary consumer A species that eats primary consumers.
Tertiary consumer A species that eats secondary consumers.
Omnivore A species that feeds at several trophic levels.
Guild Within a given trophic level, a group of species that feeds on similar items.
Within a given trophic level, we can group species that feed on similar items into guilds. For example, a grassland might contain a wide variety of primary consumers that feed on plants in different ways; these consumers can be categorized into guilds of leaf eaters, stem borers, root chewers, nectar sippers, or bud nippers. Members of a guild feed on similar items but they need not be closely related. In Chapter 16, for example, we discussed how ants and rodents in the deserts of the southwestern United States compete for seeds of the same size. Though ants and rodents are not closely related, their competition for the same seeds would place them in the same guild.
431
Direct Versus Indirect Effects
Direct effect An interaction between two species that does not involve other species.
As we have seen, because communities can include a large number of species and species interactions, a change in the abundance of any one species can affect the abundance of the other species. The simplest way that one species can affect the abundance of another is through a direct effect, which occurs when two species interact without involving other species. We have discussed a variety of direct effects in our chapters on predation, parasitism, competition, and mutualism.
Indirect effect An interaction between two species that involves one or more intermediate species.
Because so many species are interconnected in a food web, the direct effects of one species on another often set off a chain of events that continue to affect still other species in the community. When two species interact in a way that involves one or more intermediate species, we call it an indirect effect. When indirect effects are initiated by a predator, we call it a trophic cascade. Indirect effects are widespread in ecological communities. For example, in Chapter 14 we discussed how scientists introduced the Myxoma virus onto Macquarie Island to help reduce a population of European rabbits that had been introduced by sailors in the 1800s. As the virus killed the rabbits, there were fewer rabbits remaining to consume the vegetation, which resulted in an increase in the island’s vegetation. In short, the virus caused an indirect effect on the vegetation.
Trophic cascade Indirect effects in a community that are initiated by a predator.
432
Exploitative competition between two animals might at first glance seem like a direct effect because each species has a negative effect on the other. However, it is actually an indirect effect because the two competitors are interacting with each other by feeding on a common resource. In the case of the ants and rodents in the southwestern United States, for instance, the presence of ants causes a reduction in available seeds and this reduction causes the rodent population to decline. In short, the negative effect of one competitor on another is mediated through the abundance of a third species, which is a shared resource.
Indirect effects are common within communities, but sometimes they can occur between communities. For example, fish prey on aquatic insects, including the aquatic larvae of dragonflies. As a result, ponds containing fish typically have fewer dragonfly larvae. Dragonfly larvae eventually metamorphose into dragonfly adults that consume bees and flies, which are common plant pollinators. You can see this food web in Figure 18.19a. When researchers examined the dragonfly populations of ponds with fish and ponds without fish, they confirmed that the ponds without fish had more larval and adult dragonflies. Because of this higher abundance of dragonflies, the ponds without fish also had fewer bees and flies visiting flowering plants along the shoreline, as shown in Figure 18.19b. The scarcity of bees and flies reduced potential seed production in at least one plant species. Thus, a direct effect between fish and dragonflies caused a cascade of indirect effects on the surrounding terrestrial community.
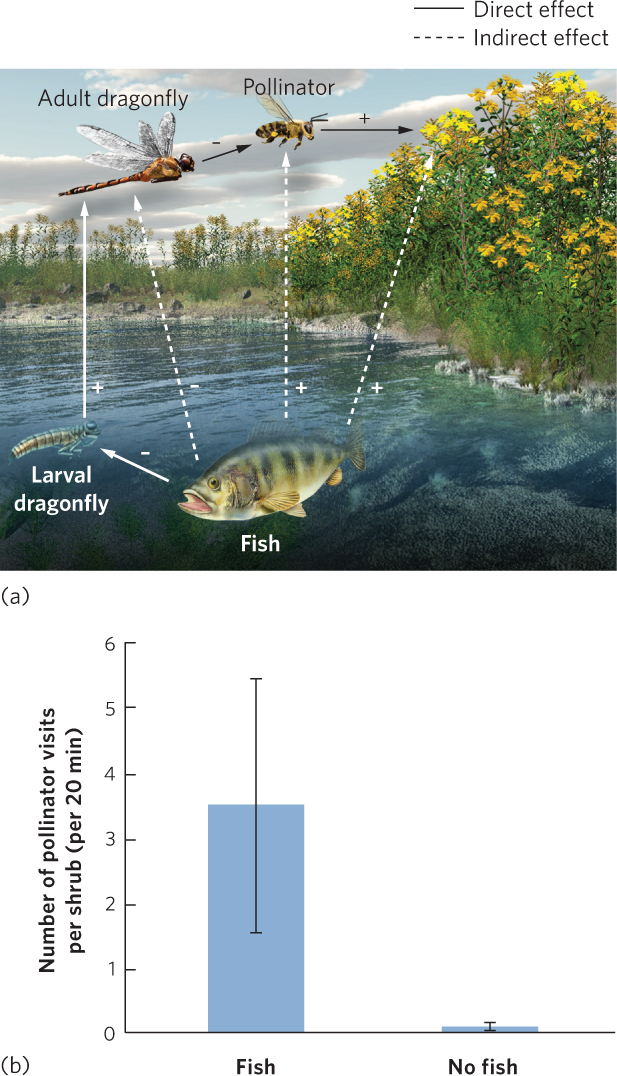
Ecologists previously assumed that indirect effects occurred only when changes in one species altered the density of another species, which initiated a trophic cascade. However, more recent research has shown that trophic cascades can also be initiated when one species causes changes in the traits of another species. We now look at a variety of indirect effects in more detail.
Density-Mediated Indirect Effects
Density-mediated indirect effect An indirect effect caused by changes in the density of an intermediate species.
When indirect effects are caused by changes in the density of an intermediate species, we call them density-mediated indirect effects. For example, increased densities of sea stars in intertidal communities cause a decline in mussels, which allows other species such as snails to occupy the limited open space on the rocks. The positive indirect effect of sea stars on snails occurs because the sea stars reduce the density of mussels. Similarly, the introduction of Myxoma virus reduced the density of rabbits on Macquarie Island, which allowed an increase in the growth of the plants that rabbits eat.
433
Trait-Mediated Indirect Effects
More recently ecologists have come to appreciate that communities can also experience trait-mediated indirect effects, which are indirect effects that are caused by changes in the traits of an intermediate species. This commonly happens when a predator causes its prey to change its feeding behavior, which in turn alters the amount of food consumed by the prey. For example, the reintroduction of wolves into Yellowstone National Park has caused elk to feed in more protected areas. This behavior alters the amount of plant consumption that occurs in these areas, not because the density of elk has changed but because the behavior of the elk has changed.
A similar example can be found in a community of spiders, grasshoppers, and grasses. Grasshoppers are insects that readily consume grasses and serve as prey for some species of spiders. There is more grass present when spiders are present in the community. The increase in grass could occur because spiders eat the grasshoppers and therefore lower the grasshopper’s density, or because the spiders scare the grasshoppers so that they hide more and spend less time feeding. To determine which process is operating, researchers conducted an experiment in which they placed cages around small areas of grass in a field and then conducted one of four manipulations by adding: (1) no animals (the control), (2) grasshoppers, (3) grasshoppers and lethal spiders, and (4) grasshoppers and nonlethal spiders. Although the nonlethal spiders could not kill the grasshoppers because their mouthparts had been glued shut, the grasshoppers would still recognize them as potential predators, which should cause grasshoppers to reduce their feeding time.
Trait-mediated indirect effect An indirect effect caused by changes in the traits of an intermediate species.
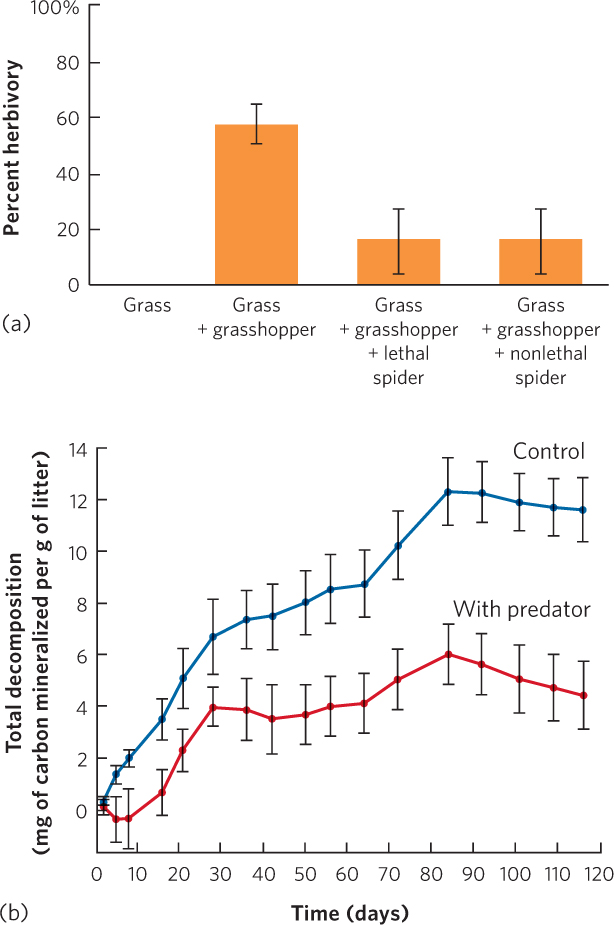
When researchers added the lethal spiders, the grass experienced less herbivory, as you can see in Figure 18.20a. At first glance, this appears to be a density-mediated indirect effect because spiders kill grasshoppers. However, the researchers found that the density of grasshoppers was not affected by the presence of lethal spiders, probably because grasshopper reproduction offset spider predation. When the researchers added nonlethal spiders with glued mouthparts, the number of grasshoppers was identical to when lethal spiders were added and the grass still experienced reduced herbivory. In short, nonlethal spiders, which can alter the behavioral traits of the grasshoppers but not grasshopper density, had the same positive indirect effect on the grass as lethal spiders, which alter both the density and the traits of grasshoppers. These results illustrated that the mere presence of spiders can alter the behavioral traits of grasshoppers and initiate a trait-mediated indirect effect.
Ecologists are beginning to discover that trait-mediated effects can be far-reaching. For example, in 2012 researchers reported that grasshoppers that are exposed to the threat of spider predation not only feed less, but their bodies also contain less nitrogen.
434
Because nitrogen is typically a limited resource in soils, as we discussed in Chapter 3, researchers suspected that the amount of nitrogen in grasshopper bodies would affect the rate of soil decomposition when grasshoppers die and become part of the detritus. To test this hypothesis, researchers again raised grasshoppers with no spiders and spiders with glued mouthparts. After being exposed to the two treatments, the grasshoppers were killed and their carcasses were mixed with dead grass. When researchers measured the rate of grass decomposition, they found that it was approximately three times faster with grasshoppers that had been reared without spiders than grasshoppers that had been reared with spiders, as shown in Figure 18.20b. These results demonstrate that trait-mediated effects can be far-reaching throughout communities.
Top-Down and Bottom-Up Effects
Bottom-up control When the abundances of trophic groups in nature are determined by the amount of energy available from the producers in a community.
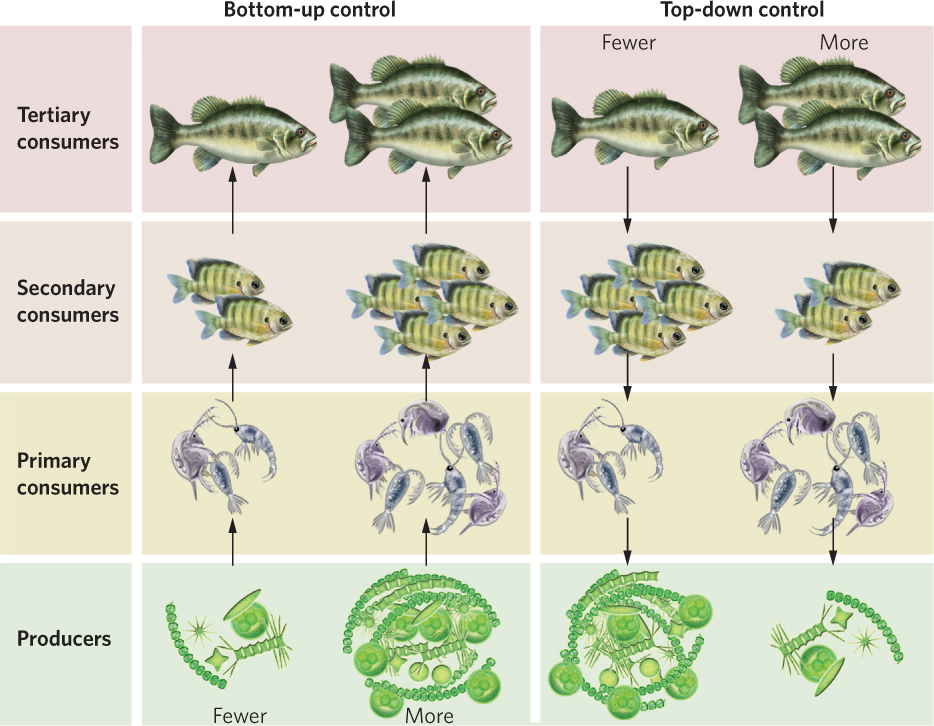
The amount of available resources as well as the amount of predation and parasitism a species experiences can affect the abundance of species. The same can be said for entire trophic groups. When the abundances of trophic groups in nature are determined by the amount of energy available from the producers in a community, we say there is bottom-up control of the community. When the abundance of trophic groups is determined by the existence of predators at the top of the food web, we say there is top-down control of the community. You can see both of these concepts illustrated in Figure 18.21. If we return to our lake example, we can start with four trophic groups that consist of large fish, small fish, zooplankton, and phytoplankton. If an increase in phytoplankton causes an increase in the zooplankton, small fish, and large fish, the abundance of the trophic groups experiences bottom-up control. If an increase in the abundance of large fish causes a decrease in the small fish, an increase in the zooplankton upon which the small fish feed, and a decrease in phytoplankton, the abundance of the trophic groups experiences top-down control.
Top-down control When the abundance of trophic groups is determined by the existence of predators at the top of the food web.
435
For many years, ecologists debated whether communities are more commonly under top-down or bottom-up control. If we think of food webs as having three broad trophic levels, top-down control by predators would reduce the abundance of herbivores and this would result in an abundance of vegetation. In a classic paper published in 1960, Nelson Hairston, Frederick Smith, and Lawrence Slobodkin suggested that because most communities contain an abundance of vegetation, trophic groups must be controlled from the top of the food web.
Their hypothesis caused a great deal of debate among ecologists and inspired researchers to survey patterns of abundance in nature and to conduct manipulative experiments. For example, when researchers surveyed the abundance of zooplankton and phytoplankton, they found that ponds with more phytoplankton also had more zooplankton, which suggested that zooplankton abundance is controlled from the bottom up. However, when they conducted manipulative experiments in which they added small fish that eat zooplankton, the abundance of zooplankton decreased, which caused a trophic cascade that allowed the phytoplankton to increase. This suggested that the abundance of species in the community was controlled from the top down. Over the past 2 decades, it has become clear that many communities are simultaneously controlled both from the top down by predators and from the bottom up by resources. For example, the number of zooplankton in a lake can be influenced both by the amount of phytoplankton available for consumption and by the number of small fish available to consume the zooplankton.