Succession occurs in a community when species replace each other over time
Succession The process by which the species composition of a community changes over time.
The process of succession in a community is the change in species composition over time. For example, when a field is plowed but not planted, it soon becomes colonized by grasses and wildflowers. In climates with sufficient precipitation, the grasses and wildflowers will eventually be replaced by shrubs and then by large trees. Each stage of community change during the process of succession is known as a seral stage. The earliest species to arrive at a site are known as pioneer species. These species typically have the ability to disperse long distances and arrive quickly at a disturbed site. The final seral stage in this process of succession is known as the climax community. A climax community is generally composed of the group of organisms that dominate in a given biome. As we will see, a climax community can be achieved through a variety of different sequences over time. Also, the climax community may continue to experience change.
Seral stage Each stage of community change during the process of succession.
Pioneer species The earliest species to arrive at a site.
Climax community The final seral stage in the process of succession.
445
We begin this section by considering two methods of observation that ecologists employ to study succession: direct and indirect. We will then examine how these techniques help scientists examine successional patterns in a variety of terrestrial and aquatic environments. While most studies of succession focus on the changes in plant communities, there are also associated changes in the species of animals that depend on plants for food and habitat. Much less studied is the succession that occurs on dead organic material such as rotting logs and animal carcasses.
Observing Succession
Observing succession in a community can take different amounts of time depending on the life histories of the species involved. For example, the succession of decomposers on a dead animal can happen in a matter of weeks or months. In contrast, the succession of a forest can take hundreds of years. When succession occurs over long time horizons, modern scientists can sometimes return to sites that have been studied by other scientists decades or even centuries earlier and observe how the species composition of an area has changed over time. In other cases, when scientists have no historical data for an area, they must use more indirect methods to estimate the pattern of succession. As we will see, taking multiple direct and indirect approaches to examine succession provides the most complete picture of how communities change over time.
Direct Observations
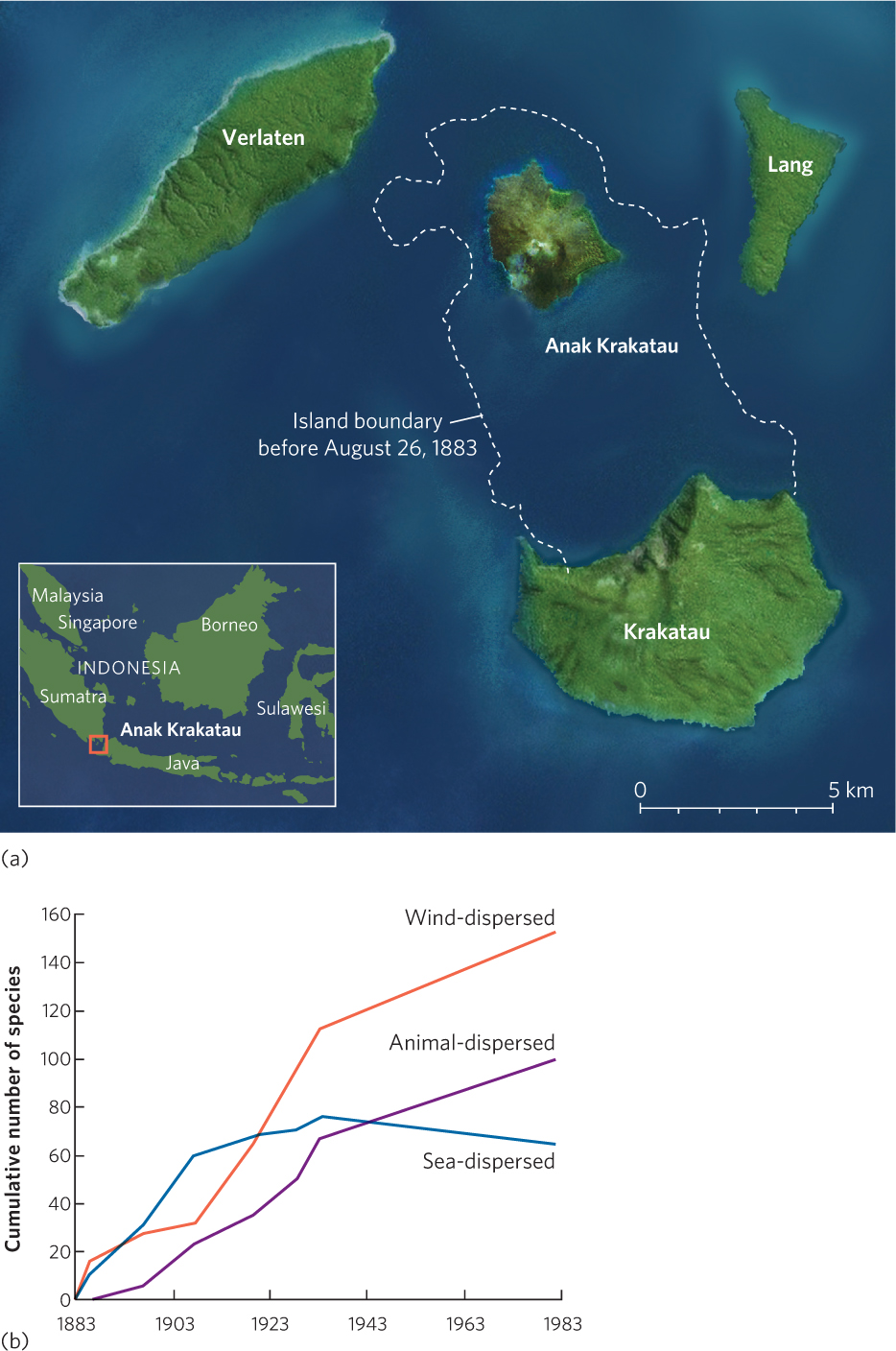
The clearest way to document succession in a community is by direct observation of the changes over time. We have already described the research at Glacier Bay, a well-known example of directly observed succession. Another example comes from the small island of Krakatau, Indonesia.
In 1883 a massive volcanic eruption on Krakatau blew away nearly three-quarters of the island, as depicted in Figure 19.1a. The remaining part of the island was covered with a layer of volcanic ash that obliterated all life. Researchers began to visit the island after the eruption to document how and when various species would return and whether the developing community would eventually be similar to the community that had existed on the island before the eruption. By 1886 the researchers observed that 24 species of plants had colonized Krakatau, as you can see in Figure 19.1b. Ten of these species were sea-dispersed plants that are common on tropical shores throughout the region. Many of the other plants were wind-dispersed grasses and ferns whose seeds and spores had been blown to the island from surrounding islands. With more time, the seeds of trees arrived. By 1920 most of the island had developed into a forest community. The presence of forest habitats made the island more hospitable to many species of birds and bats. Bird and bat species that were fruit eaters brought a variety of seeds with them in their digestive systems. Many of the seeds that were excreted while the birds and bats were on the island subsequently germinated. After the 1920s most of the new plant species on the island were animal-dispersed species brought by visiting birds and bats. Today, scientists continue direct observations to monitor how succession on the island is affected by additional disturbances such as volcanic eruptions, erosion of ash deposits by the ocean waves, and strong storms that pass through the region.
Indirect Observations
Because it is a challenge to directly observe succession in many types of communities, researchers have sought ways to determine the patterns of succession indirectly. The two most common methods attempt to look back in time from the present day. One approach examines regional communities that began succession at different times. For example, classic research by Henry Cowles in the late 1800s examined the succession of sand dunes in Indiana along the southern shore of Lake Michigan. Cowles knew that the water level in Lake Michigan had fallen since the last glaciation and that new sand dunes had formed along the edges of the receding shoreline. At the time Cowles visited Lake Michigan, the lake was surrounded by multiple ridges of sand dunes. The oldest dunes were far away from the current shoreline while the youngest dunes were close to the shoreline. On these younger dunes, he found scattered plants such as beachgrass (Ammophila breviligulata) and bluestem grasses that represented the earliest stages of succession, much like the wind-dispersed grasses that appeared on Krakatau during the earliest years of succession. Farther away from the water, older dunes contained larger and more abundant plants that included herbs and several species of shrubs. Beyond these plants, still older dunes had pine trees, while the oldest dunes had beech, oak, maple, and hemlock trees (Figure 19.2). These observations led Cowles to identify the concept of a chronosequence, which is a sequence of communities that exist over time at a given location. Chronosequences help ecologists understand how succession has progressed over time in an area. Many other ecologists subsequently used chronosequences, including William Cooper, a graduate student of Cowles who examined chronosequences in Glacier Bay.

Chronosequence A sequence of communities that exist over time at a given location.
446
It is also possible to look back in time by examining pollen and other plant parts preserved in distinct layers of lake and pond sediments. Flowering plants produce pollen grains with distinct sizes and shapes. When these pollen grains travel through the air and land on the surface of a lake, they sink and, over time, become preserved in layers of sediment at the bottom of the lake. To determine the age of each of these layers, researchers take a sample that penetrates through many layers of mud on the lake bottom and use a technique known as carbon dating that identifies the age of the pollen in each layer. Dating the pollen helps them determine changes in the species of plants around the lake over hundreds or even thousands of years.
447
SUCCESSION IN TERRESTRIAL ENVIRONMENTS
Researchers investigating terrestrial environments have focused primarily on the succession of plant communities. In terrestrial environments, we can categorize succession into two types that are based on their starting conditions: primary succession and secondary succession. In both cases, we will begin by discussing a simplified version of terrestrial succession that is represented by an ordered progression over time. Later in this section we will see that succession in terrestrial communities can be much more complex.
Primary Succession
Primary succession The development of communities in habitats that are initially devoid of plants and organic soil, such as sand dunes, lava flows, and bare rock.
Primary succession is the development of communities in habitats that are initially devoid of plants and organic soil, such as sand dunes, lava flows, and bare rock. These inhospitable environments are colonized by species that require no soil, such as lichens and mosses that can live on the surfaces of rocks, and drought-tolerant grasses that are able to colonize dry sand dunes (Figure 19.3). The species that first colonize these places produce bits of organic matter that combine with the processes of rock weathering and microbial activity to create soils that make the site more hospitable for other species.

Secondary Succession
Secondary succession The development of communities in habitats that have been disturbed and contain no plants but still contain an organic soil.
Secondary succession is the development of communities in habitats that have been disturbed and contain no plants but still have an organic soil. For example, secondary succession occurs in fields that have been plowed or forests that have been uprooted by a hurricane. Such habitats typically contain well-developed soils, plant roots, and seeds, all of which contribute to rapid growth of new plants after the disturbance.
448
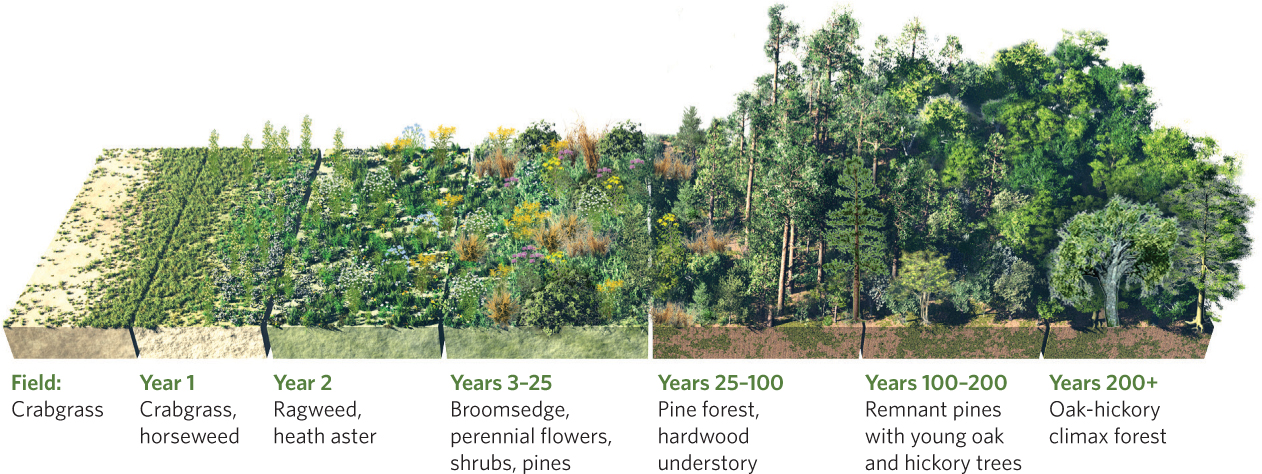
Secondary succession can be observed in a chronosequence of abandoned agricultural fields. An excellent example of this is Duke Forest, an outdoor laboratory in the Piedmont region of North Carolina composed of 1,900 ha of abandoned agricultural fields and forests that Duke University purchased in 1931. To understand the process of succession in this area, ecologist Henry Oosting visited multiple farm sites, each abandoned for different lengths of time. He found a clear pattern of succession that starts with annual plants and ends with large deciduous trees, as illustrated in Figure 19.4. Even before abandonment, crabgrass (Digitaria sanguinalis) is common in the fields. In the first summer after abandonment, crabgrass and horseweed dominate the fields. By the second summer, ragweed (Ambrosia artemisiifolia) and heath asters (Symphyotrichum ericoides) dominate the fields, and by the third summer broomsedge (Andropogon virginicus) dominates. Next come shrubs, followed by pines. Within about 25 years, pines eventually crowd out earlier successional species. As the decades pass, deciduous tree species arrive and start displacing the pines. After about 100 years, deciduous trees dominate and constitute the last seral stage in the successional sequence.
We distinguish between primary and secondary succession by the starting point of the community. For example, in the midwestern United States, an area containing bare rock will pass through seral stages of lichens and mosses, annual weeds, perennial weeds, shrubs, several species of pioneer tree species, and then a beech-maple forest. In contrast, an area containing bare soil will start with annual weeds and then pass through many of the same seral stages on its way to form a beech-maple forest. As we will see later in the chapter, this beech-maple forest can vary somewhat in the composition of dominant tree species.
Sometimes the distinction between primary and secondary succession is unclear because the intensity of a disturbance can vary. For instance, because a tornado that levels a large area of forest usually does not harm soil nutrients, seeds, and living roots, succession follows quickly. In contrast, a severe fire that burns through organic layers of the forest soil requires the community to start over from scratch in a way that resembles primary succession; there is soil, but it contains few seeds or roots that can immediately sprout after the fire.
The Complexity of Terrestrial Succession
A given biome has a characteristic climax community, but the sequence of seral stages through which a single site passes on its way to this climax community can differ depending on the initial conditions. For example, consider the succession of a field, a sand dune, and a wetland near Lake Michigan in Indiana. The abandoned field is typically colonized by asters that include horseweed and goldenrod. The sand dune is typically colonized by beachgrass and bluestem grasses, which are perennial grasses that can stabilize the sand dunes and add organic matter to the soil. The wetland supports plants such as cattails, which produce organic matter; over many years this organic matter can fill in the wetland and create conditions that allow terrestrial plants to colonize the site. In all three cases, the progression of seral stages begins with a different community but ends with the same climax community of a forest dominated by beech and maple trees.
449
Although it is tempting to think that terrestrial succession is a simple linear process, modern studies have demonstrated that this is often not the case. The sequence of seral stages can be quite variable. For example, the concept of chronosequences helps ecologists observe how a community changes in space and over time. However, this approach relies on the assumption that older sites pass through the same stages as the younger sites. It also assumes that sites of different ages do not differ in other aspects, such as historic abiotic conditions, soil fertility, and human or natural disturbances. When chronosequences go back hundreds of years, it can be difficult or impossible to confirm that the sites did not differ in ways that affect succession. For example, researchers have found that sites of similar age in a given area sometimes contain important differences in species composition because of local disturbances such as a tornado. Modern researchers will observe the changes in species composition, but they may not know that a tornado had passed through the area.
The research at Glacier Bay provides a good example of the complexity of succession. For decades, ecologists presented a simplified scenario of how the communities in Glacier Bay have changed over time. Based on Cooper’s original observations, they identified primary succession as a simplified linear process that began with lichens, mosses, and herbs. Next is a seral stage containing low shrubs, followed by a stage containing tall shrubs that include willows, cottonwoods, and alders. The next stage is dominated by spruce trees and the final seral stage is dominated by hemlock trees.
More recent research has used tree rings to date the colonization of successional sequences and has reached a different conclusion regarding the path of succession. The number of growth rings tells us the age of the tree. From the tree’s age, we can determine when the tree first appeared at a site relative to when the retreating glacier opened the site to succession. For example, we now know that the older sites probably never had a seral stage that included cottonwood trees, whereas the younger sites currently pass through a stage of abundant cottonwoods. Furthermore, spruce and hemlock trees rapidly colonized sites that were exposed in the early 1800s, but not sites that were exposed in the late 1800s and early 1900s. You can view the data for Sitka spruce trees in Figure 19.5. This suggests that the soils in the older and younger sites may not have experienced the same changes as they underwent succession.

Because the assumptions underlying chronosequences may not always be supported, the best approach is to use a combination of research methods including chronosequences, pollen records, and long-term studies of single locations undergoing succession. Collectively, these studies provide the most accurate description of terrestrial succession.
450
Animal Succession
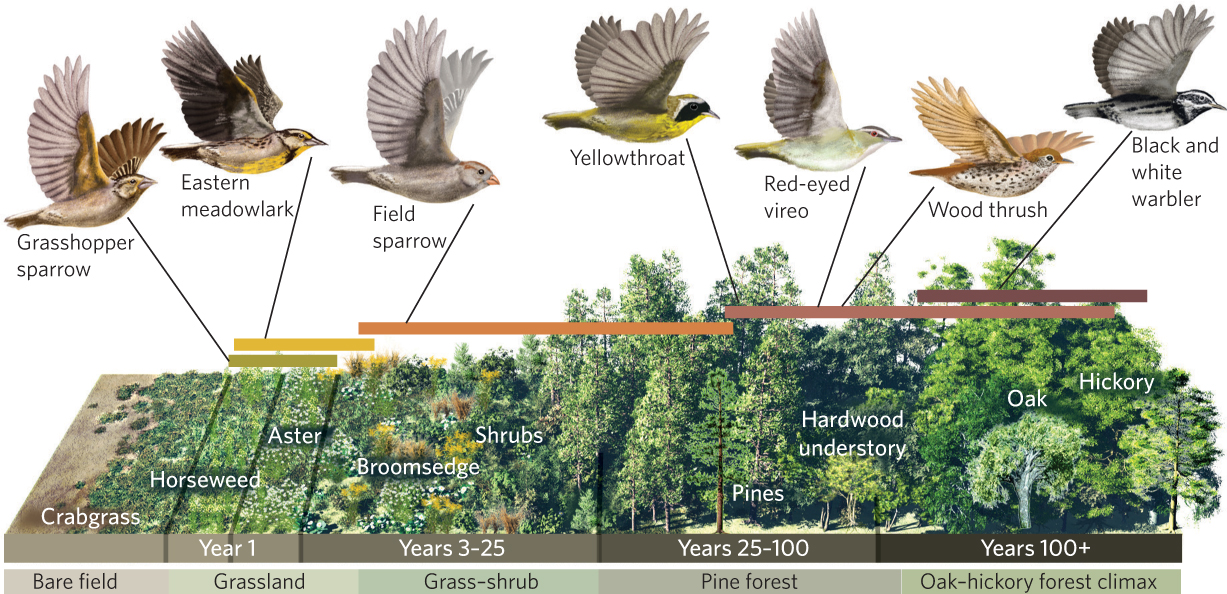
Ecologists have traditionally focused on changes in plant species when describing succession in terrestrial environments. However, the changes in the plant community cause substantial changes in the habitats that are available to animals, which in turn cause changes in the animal community. For example, a classic study by David Johnston and Eugene Odum examined the distribution of bird species in the Piedmont region of Georgia, along the same successional seral stages that Oosting surveyed in the Piedmont region of North Carolina. As illustrated in Figure 19.6, grasshopper sparrows (Ammodramus savannarum) and eastern meadowlarks (Sturnella magna) dominate the early-succession stages that contain annual plants. With the colonization of shrubs into the abandoned fields comes the arrival of many different bird species, including field sparrows (Spizella pusilla) and yellowthroats (Geothlypis trichas). As we move from the pine forest to the oak-hickory climax forest, other species appear that include the red-eyed vireo (Vireo olivaceus) and the wood thrush (Hylocichla mustelina). Although some species of birds specialize on a narrow range of the plant successional stages, many species inhabit multiple seral stages.
Succession in Aquatic Environments
Succession also occurs in aquatic environments. In this section, we will examine how succession proceeds in three types of aquatic environments: intertidal zones, streams, and ponds.
Intertidal Communities
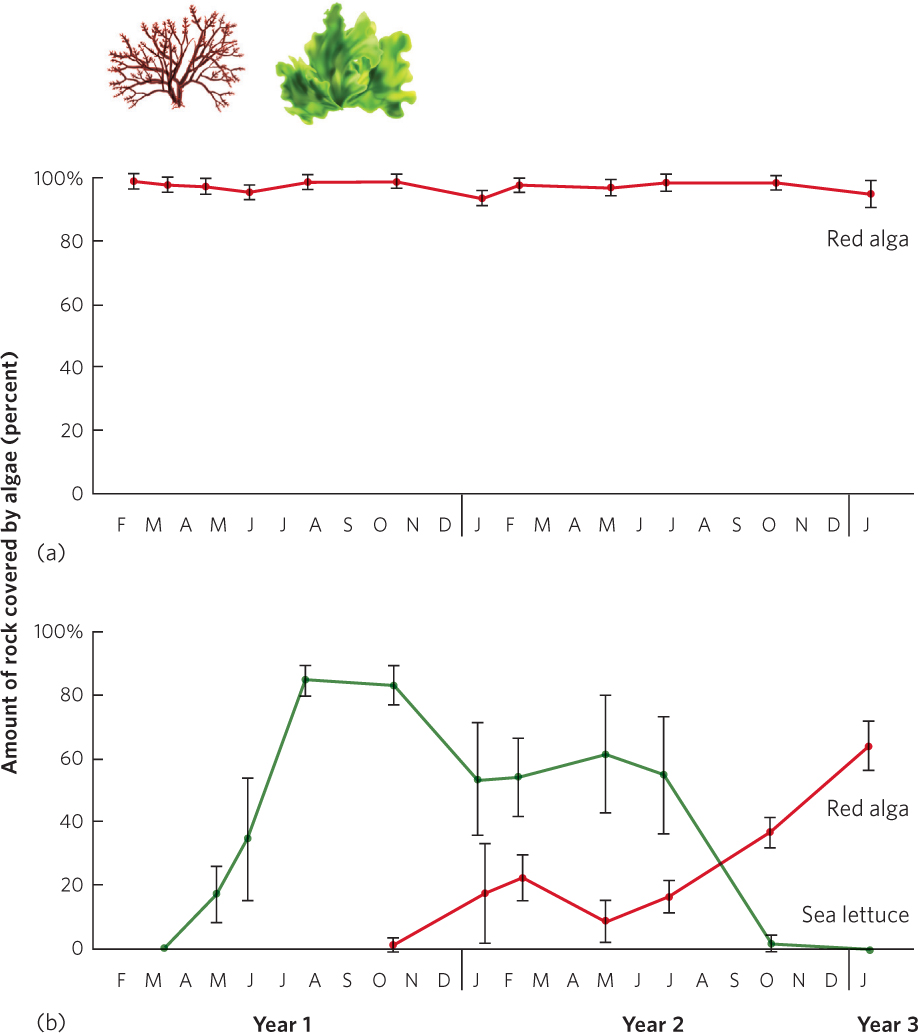
In contrast to most terrestrial communities, succession in intertidal communities can occur much faster after a disturbance, in part because the generation time of the dominant species is much shorter. In intertidal communities, powerful waves that occur during storms commonly remove organisms that are attached to boulders. For example, ecologist Wayne Sousa in a classic experiment examined the succession of different species of algae on boulders of the intertidal zone in southern California. He examined some boulders that were not overturned by storms and others that had been overturned and had areas of bare rock exposed that could be colonized by algae. As shown in Figure 19.7, Sousa observed that the first species to arrive was a green alga known as sea lettuce (Ulva lactuca). Over the next year, sea lettuce came to dominate the rocky habitat and largely prevented a competing species of red alga, Gigartina canaliculata, from colonizing. However, as the sea lettuce became more dominant, it attracted crabs that eat it, which cleared areas on the boulders for the less edible red algae to colonize. Over time, red algae came to dominate the community.
Stream Succession
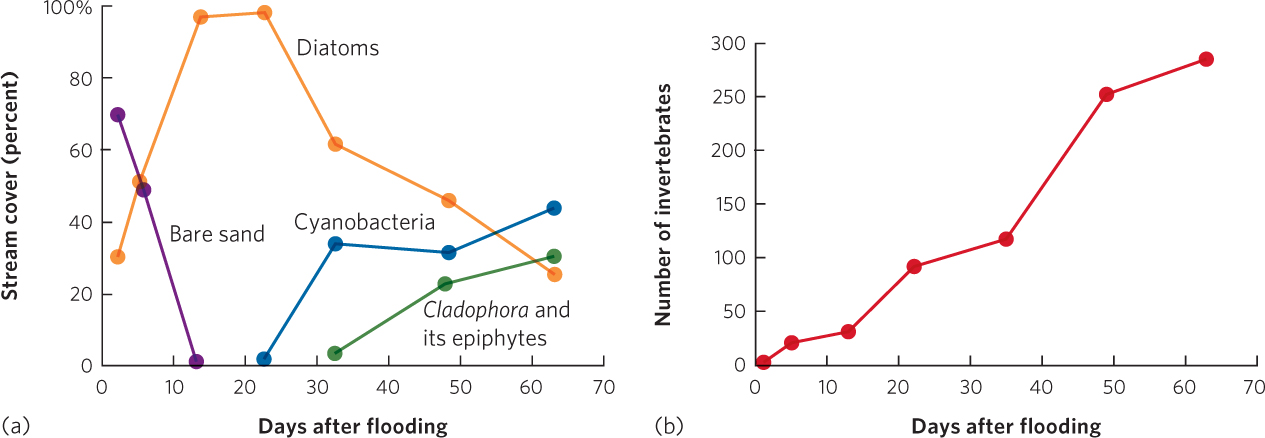
Like intertidal habitats, streams also experience rapid succession largely because organisms can move downstream from sites that are less disturbed. Streams can experience major disturbances during heavy rainfall that increases both the volume of water the streams carry and the speed at which the water moves. With the greater speed of the rushing water, sand and rocks can tumble downstream and wipe out most plants, animals, and algae. In one study, researchers looked at the effects of a flood event in Sycamore Creek in Arizona. The floodwater scoured the creek and eliminated nearly all of the algae and 98 percent of the invertebrates, leaving behind rocks and bare sand. The researchers then monitored how the community changed over the subsequent 2 months; you can view their data in Figure 19.8. Within just a few days after the flood, the stream became colonized with several species of algae known as diatoms. Within 5 days, the diatoms covered nearly 50 percent of the creek bottom and after 13 days the diatoms covered nearly 100 percent. After 3 weeks, cyanobacteria started to colonize the stream, followed by a species of filamentous green alga (Cladophora glomerata) along with associated diatoms that live as epiphytes on this green alga. As the three types of algae rebounded, adult insects from the surrounding terrestrial environment began to lay eggs in the stream. This returned a diverse group of larval insect species to the stream, which remained there until they metamorphosed into terrestrial adults.
451
Lake Succession
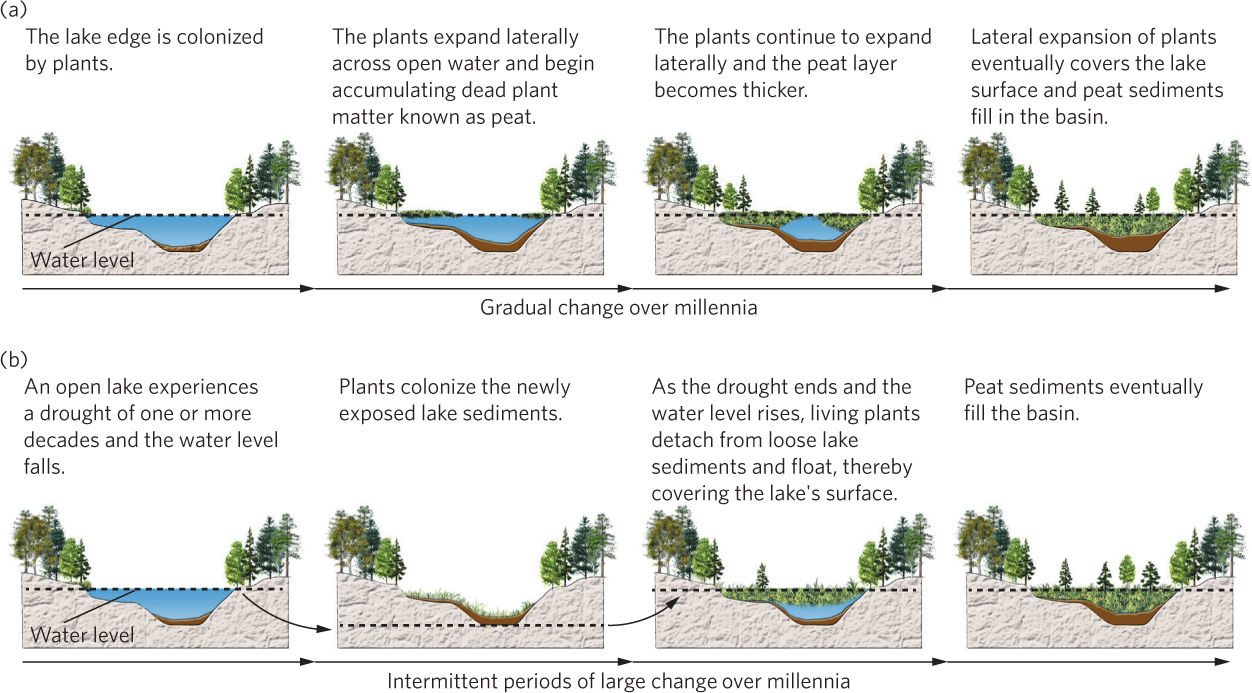
For decades ecologists have explained pond and lake succession using a paradigm of slow transformation, as depicted in Figure 19.9a. If we imagine a pond or shallow lake created by a receding glacier thousands of years ago or a beaver dam converting a stream into a pond, we start with a basin full of water. Over time, the erosion of soil and the growth and death of organisms in the lake form sediments that gradually fill the basin. In addition, plants living along the shoreline slowly extend themselves out into the water to form a floating mat of vegetation. Underneath this live mat of vegetation is an accumulating layer of partially decomposed vegetation known as peat. Eventually, the entire basin has a floating mat of vegetation that continually contributes detritus to the peat layer in the basin. Microbial decomposition of the dead vegetation is slow because the water underneath the floating mat has little oxygen. As a result, detritus accumulates on the bottom of the basin, and over hundreds or thousands of years the pond or lake becomes a bog. In North American bogs, sphagnum moss, sedges, and shrubs—such as leatherleaf and cranberry—become established along the edges and add to the development of a soil with progressively more terrestrial qualities. At the edges of the bog, shrubs may be followed by black spruce (Picea mariana) and tamarack trees (Larix laricina), which eventually give way to birch, maple, and fir trees, depending on the locality. Ecologists once believed that this succession reflects the way that many aquatic habitats are very gradually transformed—organic detritus accumulates until the soil rises above the water table and a terrestrial habitat emerges. Not all lakes experience succession into terrestrial habitats, but it is common for shallow lakes and ponds to do so over long periods of time.
452
453
ANALYZING ECOLOGY
Quantifying Community Similarity
When ecologists examine the species living in different communities, they often quantify composition and richness. Although such data tell us about the species living in each community, they do not provide a measure of comparison. To address this need, ecologists have developed several indices of community similarity that can range from zero to one; a value of zero indicates that two communities have no species in common, whereas a value of one indicates that two communities have an identical composition of species.
One of the most common ways to quantify similarity is Jaccard’s index of similarity, developed by the Swiss botanist Paul Jaccard in 1901. Jaccard’s index is calculated using the following equation:
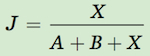
where A represents the number of species that are only present in Community A, B represents the number of species that are only present in Community B, and X represents the number of species present in both communities. For example, consider the table below that lists the species of fish found in each of three stream communities that are at different stages of succession.
We can now use Jaccard’s index to calculate the similarity between Community A and Community B.
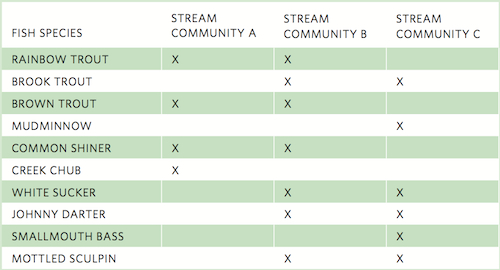
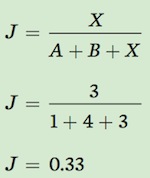
This value indicates that there is relatively low similarity in the species composition of Communities A and B.


454
In 2012, researchers proposed a new model of pond and lake succession. This model is based on studies that took core samples of a bog. By using carbon dating, researchers were able to identify when different species appeared. Contrary to the classic model of slow and continuous succession, they discovered that ponds and lakes can experience long periods of several hundred years in which little succession occurs, followed by brief episodes of rapid change. During times of prolonged drought that last for a decade or more, water levels drop and vegetation grows down onto the newly exposed shoreline. As you can see in Figure 19.9b, when the drought ends and the water level rises again, the mat of vegetation releases its hold from the lake bottom and floats up to the water’s surface. With continued growth of the vegetation, this floating mat becomes thicker and deposits dead organic matter into the water below it. For example, after studying a 16-ha bog in Pennsylvania, the researchers estimated that 50 percent of the former lake became covered by bog vegetation in just a few decades during a severe drought in the late sixteenth century. In short, lake succession does not always have to be slow and steady; it can happen in brief bursts during rare periods of drought.
Change in Species Diversity
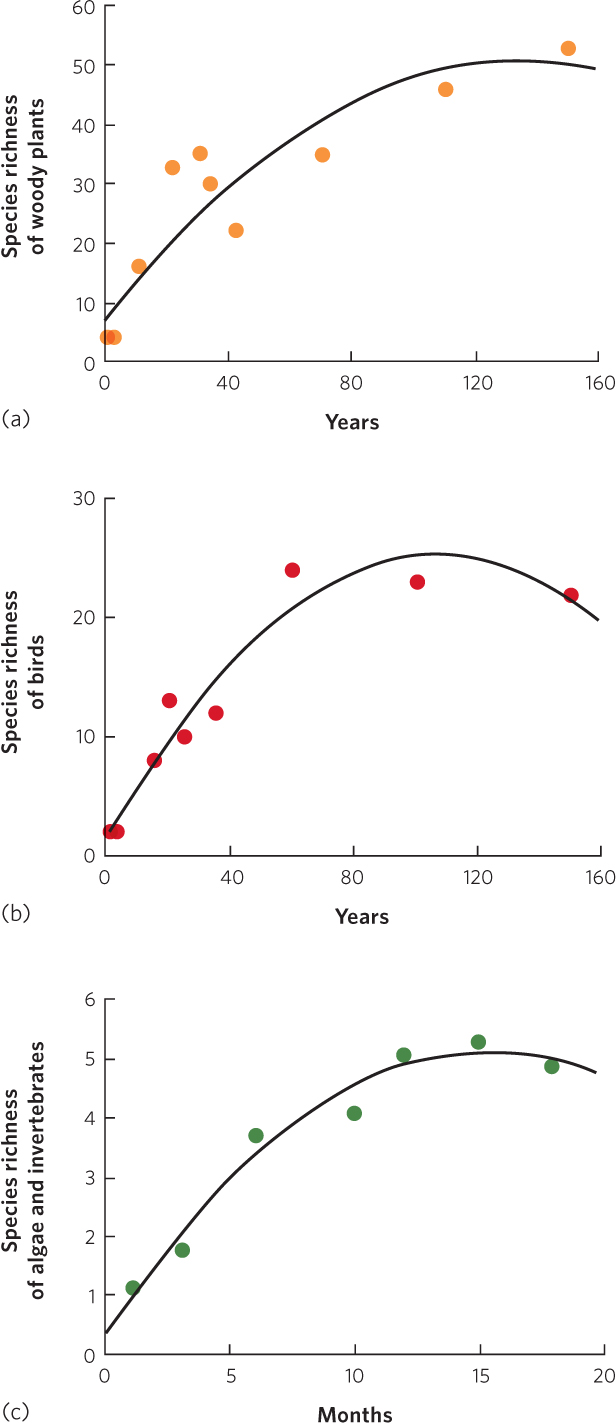
Across both terrestrial and aquatic habitats, the process of succession exhibits consistent effects on species richness. When succession follows a major disturbance that eliminates most or all of the species that had been present in a community, species richness necessarily starts at or near zero and then must increase over time with succession. In most cases of succession, we see species richness increase rapidly at first, followed by a plateau and a small decline, as shown in Figure 19.10. Oosting’s survey of the Duke Forest, for example, found a rapid increase in the species richness of woody plants during the first 25 years and then a gradual decline in the rate of increase over the next 125 years. Similar patterns of richness over time can be seen in the number of bird species observed by Johnston and Odum in a forest community and the number of algae and invertebrates observed by Sousa in an intertidal community.