Sexual selection favors traits that facilitate reproduction
Sexual selection Natural selection for sex-specific traits that are related to reproduction.
We have seen that a male’s reproductive success is typically determined by the quantity of females he can breed, whereas a female’s reproductive success is typically determined by the quality of males that can fertilize her limited number of eggs. This means that females should generally be the choosier sex. But what exactly should she be choosing? In broad terms, females should select those males that improve her fitness by the greatest amount. She might select the male with the best genotype or the male with the most resources for her and her offspring.
Because females are choosy, males should compete strongly with other males for the opportunity to breed. This intense competition among males for mates has resulted in the evolution of male traits that are either used to attract females or used in contests and combat among males. Natural selection for sex-specific traits that are related to reproduction is referred to as sexual selection. In this section, we will explore how males and females have evolved different traits as a result of sexual selection, what traits females prefer in their mates, and how the fitness interests of males and females can cause conflict between the sexes.
Sexual Dimorphism
Sexual dimorphism The difference in the phenotype between males and females of the same species.
Primary sexual characteristics Traits related to fertilization.
One result of sexual selection is sexual dimorphism, which is a difference in the phenotype between males and females of the same species, such as that observed in the honeybees at the beginning of this chapter. Sexual dimorphism includes differences in body size, ornaments, color, and courtship behavior. Traits related to fertilization—such as gonads—are referred to as primary sexual characteristics while traits related to differences in body size, ornaments, color, and courtship are known as secondary sexual characteristics.
Secondary sexual characteristics Traits related to differences between the sexes in terms of body size, ornaments, color, and courtship.
224
Sexual dimorphism can evolve due to differences in life history between the sexes, contests between males, or mate choice by females. Body size differences are common between the sexes of many animals because there has been selection for an increased number of gametes produced or for an increase in parental care by one sex. In fish and spiders, for instance, egg production is directly related to body size. This selection for greater gamete production in females without concurrent selection for greater sperm production in males could be the underlying cause of the larger size of females than males in many species of spiders and fish (Figure 9.16).

Sexual dimorphism can also occur when males compete for mates. In this case, selection will favor the evolution of weapons for combat. Such weapons include the antlers of male elk, the horns of mountain sheep, and the leg spurs of chickens and turkeys. When fighting ability is also improved by having a large body, contests between males can also favor the evolution of larger male bodies (Figure 9.17). Males that win such contests are more likely to gain access to females. Sexual dimorphism also commonly arises when one sex is choosy when selecting a mating partner. Since the females are the choosy sex in most species, female selection of males with particular traits can cause the sexual selection for extreme traits.

The Evolution of Female Choice
A female’s preference for particular male traits should be tied to those features that improve her fitness. In terms of broad categories, we can consider two types of female preferences: material benefits and nonmaterial benefits.
Material benefits are those physical items that a male can provide a female including a site for raising offspring, a high-quality territory, or abundant food. In these cases, the benefit to the female is straightforward—a site for raising offspring and resources for producing eggs and feeding the offspring should improve a female’s fitness.
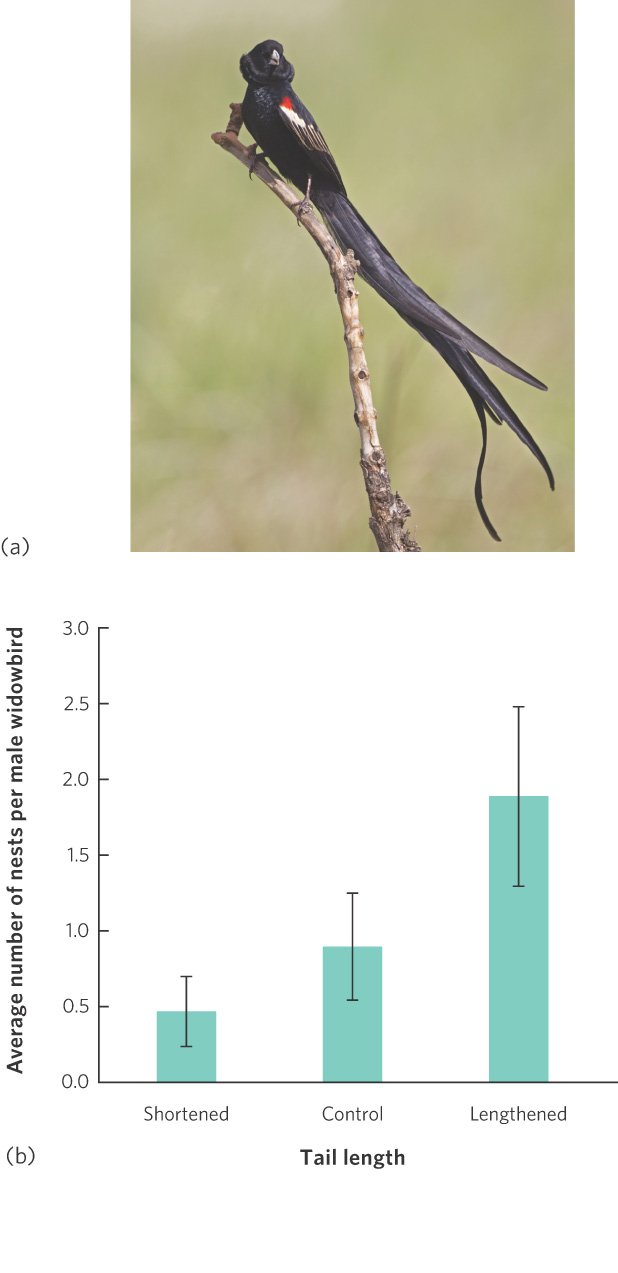
It has been more of a challenge for scientists to understand female choices when the female does not receive any material benefits from males. If the female choice is an adaptation, then there must be some benefit. One of the first demonstrations of female choice in nature for male traits that provided no material benefit came from a study of tail length in male long-tailed widowbirds (Euplectes progne), a small, polygynous species that inhabits the open grasslands of central Africa. The females are mottled brown, short-tailed, and drab. In contrast, during the breeding season males are jet black with a red shoulder patch, and they sport a half-meter-long tail that is conspicuously displayed in courtship flights (Figure 9.18a). The most successful males may attract up to a half dozen females to nest in their territories, but the males provide no care for their offspring. To determine what the females are choosing, researchers cut the tail feathers of some males to shorten them and glued the clipped feather ends onto the feathers of the tails of other males to lengthen them. As you can see in Figure 9.18b, males with experimentally elongated tails attracted significantly more females than those with shortened or unaltered tails. This suggests that even though tail length has no effect on a male’s ability to maintain a territory, females choose mates on the basis of tail length.
Good genes hypothesis The hypothesis that an individual chooses a mate that possesses a superior genotype.
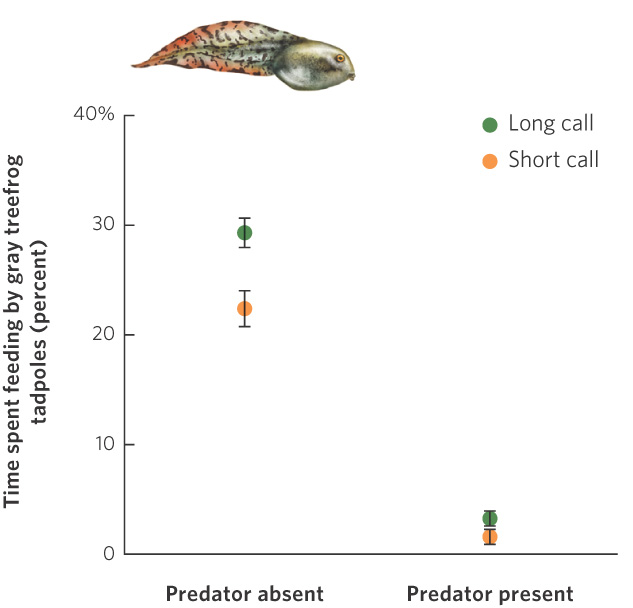
Why would females choose a male based on a trait such as the length of his tail? According to the good genes hypothesis, an individual chooses a mate that possesses a superior genotype. In the gray treefrog, for example, females prefer males that can produce the longest mating calls. Long calls can only be produced by the largest and healthiest male frogs. If male size and health have a large genetic component, choosing this trait might benefit the female’s offspring. Indeed, when researchers forced females to breed with both long- and short-calling males, the offspring of long-calling males grew faster than the offspring of short-calling males. Subsequent research, illustrated in Figure 9.19, found that the offspring of long-calling males grew faster because they spend more time feeding compared to the offspring of short-calling males.
Good health hypothesis The hypothesis that an individual chooses the healthiest mates.
225
According to a second hypothesis, known as the good health hypothesis, individuals choose the healthiest mates. Good health could be the outcome of either superior genetics or a superior upbringing with abundant resources. As a result, the good genes hypothesis and the good health hypothesis are not mutually exclusive. Females might prefer healthy males because such males may be both genetically superior and pose a lower risk of passing on a number of different parasites and diseases.
Runaway Sexual Selection
Runaway sexual selection When selection for preference of a sexual trait and selection for that trait continue to reinforce each other.
Once female preference for a male trait has evolved, the trait may continue to evolve over time. For example, if females prefer longer tails in their mates, and there is genetic variation to select from, longer tails will continue to evolve in males. When selection for preference of a sexual trait and selection for that trait continue to reinforce each other, the result can be runaway sexual selection. Runaway sexual selection is thought to have favored the evolution of such extreme traits as the half-meter-long tails of the male widowbird, the giant tail fans of the peacock, and other large male ornaments such as horns, tusks, and antlers. Runaway selection continues until males either run out of genetic variation for the trait or until the fitness costs of possessing extreme traits begin to outweigh the reproductive benefits.
226
The Handicap Principle
The handicap principle The principle that the greater the handicap an individual carries, the greater its ability must be to offset that handicap.
If sexually selected traits indicate intrinsic attributes of male quality—at least initially, before runaway sexual selection occurs—we are then faced with a paradox. Presumably, extreme traits burden males by requiring energy and resources to maintain them, and they make males more conspicuous to predators. If this is the case, it is hard to imagine how extreme traits indicate a higher quality mate.
One intriguing possibility is that elaborate male secondary sexual characteristics act as handicaps. If a male can survive with sexual traits that require extra energy to build or that increase the risk of predation, these traits might signal a superior genotype. This idea, known as the handicap principle, argues that the greater the handicap an individual carries, the greater its ability to offset that handicap with other superior qualities.
One factor that might attract females to certain males is a genetically based, high resistance to parasites and pathogens. As you know, parasites evolve rapidly and thereby continually apply selection for genetic resistance in the host. Because parasites and pathogens can impair the construction of secondary sex characteristics, a male bird that has an elaborate and showy plumage might communicate to females that because he has the energy to build elaborate feathers, he can resist parasites and pathogens. If this resistance can be inherited by the offspring of the males, then the secondary sex characteristics are honest signals of the genetic superiority of the males and indicate that only individuals with superior genes could resist parasites and maintain a bright and showy plumage.
Numerous studies have found that parasites and pathogens affect male attractiveness. In rock doves (Columba livia)—also known as pigeons—hatchling birds can be infected by mites (Dermanyssus gallinae) that live in the nest. To determine the effect of the mites on the young doves, researchers fumigated one set of infected nests to kill the mites but did not fumigate a second set of infected nests. The nestlings living in the nests with mites experienced lower survival and slower growth as a nestling (Figure 9.20). Other researchers that have examined the effects of lice on rock doves have found that females prefer louse-free males to louse-infested males by a ratio of three to one. In ring-necked pheasants (Phasianus colchicus), females prefer males with longer spurs on their lower legs. Longer spurs are linked genetically to major histocompatibility complex (MHC) genes that influence susceptibility to disease. Males with longer spurs have MHC alleles that are linked to longer life spans. Therefore, females that choose to mate with long-spurred males should produce offspring with a higher chance of surviving to reproduce as adults.

Sexual Conflict
Mating decisions were once thought to serve the mutual interest of both participants. More recently, scientists have come to appreciate that mating partners often behave according to their own self-interest. In lions, for example, when a new dominant male takes over the pride, he often kills newborn cubs that have been fathered by the previous dominant male so that the female loses her entire reproductive effort. The male obtains a fitness benefit because females without newborn cubs come into breeding condition more rapidly, which allows the new dominant male to father offspring sooner.
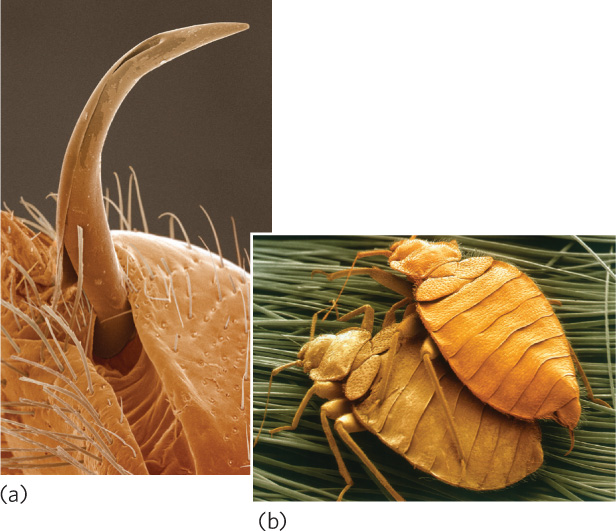
One of the more dramatic examples of sexual conflict occurs in bedbugs (Cimex lectularius). The male bedbug has a sharp sperm-transferring appendage and fertilizes a female by stabbing her with the appendage and injecting his sperm into her circulatory system (Figure 9.21). These sperm make their way to the female’s ovaries. Females fertilized by several males live shorter lives and lay fewer eggs. It is hypothesized that this aggressive behavior evolved because female bedbugs resisted copulation attempts by male bedbugs. Examples such as these demonstrate that sexual interactions can reflect different decisions that represent the self-interest of males and females.
We have seen the ecological conditions that favor sexual versus asexual reproduction as well as the factors that favor balanced versus biased sex ratios. We have also seen how the different investments into eggs versus sperm, as well as the need to care for offspring, have shaped the evolution of different mating systems and the evolution of secondary sex characteristics. The evolution of sex remains an active area of research and there is still much to be learned. Today, researchers continue to make new discoveries and our understanding of sex evolution continues to grow.
227
ECOLOGY TODAY CONNECTING THE CONCEPTS
MALE-HATING MICROBES
Throughout this chapter, we have seen how organisms have evolved a wide range of reproductive strategies. These strategies are typically adaptive; they improve an individual’s fitness under particular ecological conditions. We have considered the different mating strategies as the result of the individual’s genes and its environment. However, there is a growing realization that bacteria and other microorganisms living inside an organism can control its reproductive strategies.
One of the most widespread groups of sex-altering bacteria in insects belongs to the genus Wolbachia. Though first discovered nearly a century ago living in mosquitoes, it was not until 1990 that scientists realized that the bacteria could fundamentally change an individual’s reproductive strategies. Wolbachia infects a wide variety of invertebrates including spiders, crustaceans, nematodes, and insects. In fact, it is currently estimated that 70 percent of all insect species experience infections by this bacteria, which can live throughout the tissues of its host. In 2007 researchers discovered that the parasite’s entire genome has even been incorporated into the genome of the tropical fruit fly (Drosophila ananassae). An important aspect of Wolbachia’s life history is that it is only passed down to offspring via the mother’s infected egg. From the microbe’s perspective, therefore, the host’s female offspring are important for improving the microbe’s fitness whereas the host’s male offspring are useless.
228
To improve its fitness, Wolbachia has evolved ways to exploit or eliminate these useless males. In mosquitoes, Wolbachia alters sperm to prevent them from fertilizing the eggs of uninfected females, which ensures that subsequent generations will be dominated by offspring of infected females that carry the bacteria. When researchers treated wasps with an antibiotic, they discovered that Wolbachia causes parthenogenesis and prevents some species from producing males. Once the bacteria were gone, the wasps no longer underwent parthenogenesis. Scientists now wonder if parthenogenesis in other species might also be the result of a similar bacterium rather than an adaptive strategy.
In woodlice (Armadillidium vulgare), Wolbachia converts males into females by suppressing male hormones. This ability for hormones to determine sex in the embryos is similar to the effects of hormone manipulations by scientists investigating temperature-dependent sex determination in Jacky dragons that we saw earlier. In other species of invertebrates, the bacterium simply kills young males and prevents them from entering the adult population. In species with adults of both sexes, such as the Ugandan butterfly (Acraea encedon), the increase in females relative to males has caused a reversal in the typical sex roles; females now court males and compete with other females for breeding opportunities. Note that none of these sex-determining mechanisms, changes in offspring sex ratio, or mating systems has been selected as adaptive strategies of the host. Rather, they are in the best interest of the bacteria.
Wolbachia are not the only bacteria with such effects. Bacteria in the genus Rickettsia are well-known for causing diseases such as typhus and Rocky Mountain spotted fever, but other species in this genus commonly occur in invertebrates without causing disease. In 2011, researchers reported that they found a species of Rickettsia living in the sweet potato whitefly (Bemisia tabaci). In the southwestern United States, infection rates increased from 1 percent in 2000 to nearly 100 percent by 2009. This incredibly rapid spread of the bacterium occurred because infected female flies nearly doubled their reproductive output, the survival rate of their offspring increased, and the offspring sex ratio was altered from 50 percent daughters to nearly 85 percent daughters. This is interesting from the standpoint of host–parasite interactions and the evolution of reproductive strategies. It is also important for agriculture. The sweet potato white fly is a pest that sucks the sap out of the leaves of many crops including cotton, vegetables, and ornamental plants. Scientists worry that the massive increase in infection rates by Rickettsia will lead to a massive population increase of this pest.
Collectively these studies suggest that there is much more to the evolution of sex than we currently know, and that understanding the evolution of sex in these organisms can have important implications for humans, including the number of pests attacking our food supply.
SOURCES: Himler, A. G., et al. 2011. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 332: 254–256.
Knight, J. 2011. Meet the Herod bug. Nature 412: 12–14.