LIVING WITH THE DISEASE
Growing up, Emily was scarcely aware of her own disability. The visits to doctors and periodic stays in the hospital were just a part of life. All her teachers and friends knew that she had CF. “My family and friends were all so supportive,” she says. In high school she played volleyball, basketball, and soccer, and participated in many walkathons to raise money for CF research. Thanks to medical progress, Emily has outlived doctors’ original expectations by almost two decades.
237
But she deals daily with the legacy of her genetic inheritance. In healthy people, the CFTR protein acts as a channel through a cell’s membrane that allows certain ions to move in and out of the cell, keeping the cell’s chemistry in balance. But in people with CF, the channel is distorted or absent altogether, and the mechanism goes awry. The result is that mucus—a slippery substance that lubricates and protects the linings of the airways, digestive system, reproductive system, and other tissues—becomes abnormally thick and sticky.
This abnormal mucus blocks ducts throughout the body. The most problematic symptom, however, is that thick mucus builds up in the lungs. Patients have trouble breathing, and the mucus provides fertile ground for bacteria and other organisms. Over time, repeated infections permanently damage the lungs. As a result, people with CF may slowly lose their ability to breathe, eventually dying of suffocation (INFOGRAPHIC 11.6).

Emily remains undaunted. “I just live each day at a time,” she says. She works about 30 hours a week at a retail shop in downtown Detroit, runs to stay fit, and spends her evenings practicing with her band, performing at concerts, playing guitar, or hanging out with friends. She hopes her band’s fame and success will grow—if the band’s following expands beyond Detroit, she hopes to tour Europe. Emily hasn’t ruled out having a family of her own one day. Even though she has CF, her children will not necessarily have the disease.

RECESSIVE ALLELE An allele that reveals itself in the phenotype only if a masking dominant allele is not present.
238
DOMINANT ALLELE An allele that can mask the presence of a recessive allele.
Why not? Remember that since Emily has CF, her parents, Lowell and Debbie, both must carry disease alleles. But as neither of them has the disease, the CF alleles must be “hidden.” When one allele masks the effect of another, the hidden allele is described as recessive (designated by a lower-case letter, e.g., a). The normal allele, which conceals the effect of the recessive allele, is known as the dominant allele (designated by a capital letter, e.g., A). Debbie and Lowell are healthy because they each have a dominant normal allele that compensates for their defective recessive CF allele. Geneticists call their genotype heterozygous. Their two healthy sons are either heterozygous like their parents, or have two normal alleles—that is, their genotype is homozygous for the normal allele. A genotype made up of two dominant alleles is known as homozygous dominant. Emily’s genotype, however, is homozygous recessive: she inherited one recessive CF allele from each parent, which is why she has the disease.
HETEROZYGOUS Having two different alleles.
HOMOZYGOUS Having two identical alleles.
PUNNETT SQUARE A diagram used to determine probabilities of offspring having particular genotypes, given the genotypes of the parents.
CARRIER An individual who is heterozygous for a particular gene of interest, and therefore can pass on the recessive allele without showing any of its effects.
What were the chances that Debbie and Lowell would have a child with CF? To figure out the likelihood that parents will have a child with a particular trait, we can plot the possibilities on a Punnett square (a tool named for the geneticist Reginald C. Punnett, who devised it). A Punnett square matches up the possible parental gametes and shows the likelihood that particular parental alleles will combine. As heterozygous individuals, Debbie and Lowell each have a 50% chance of passing on their CF allele to a child, which means they have a 25% chance of having a child with CF and a 75% chance of having a healthy child. The chance that a child will be a heterozygous carrier—that is, that the child will carry the recessive allele for CF but will not have the disease because the allele’s effect is masked by the dominant allele—is 50% (INFOGRAPHIC 11.7).
Cystic fibrosis is a recessive trait, which means that the disease phenotype is caused by inheriting two recessive alleles, as in Emily’s case. Emily’s parents do not have CF because they each possess one dominant allele, but they each also carry one recessive CF allele, making them both heterozygous carriers. To calculate the probability that Debbie and Lowell will have a child with CF, we can determine the possible alleles in their gametes and then join all possible combinations of these sperm and egg in a Punnett square.
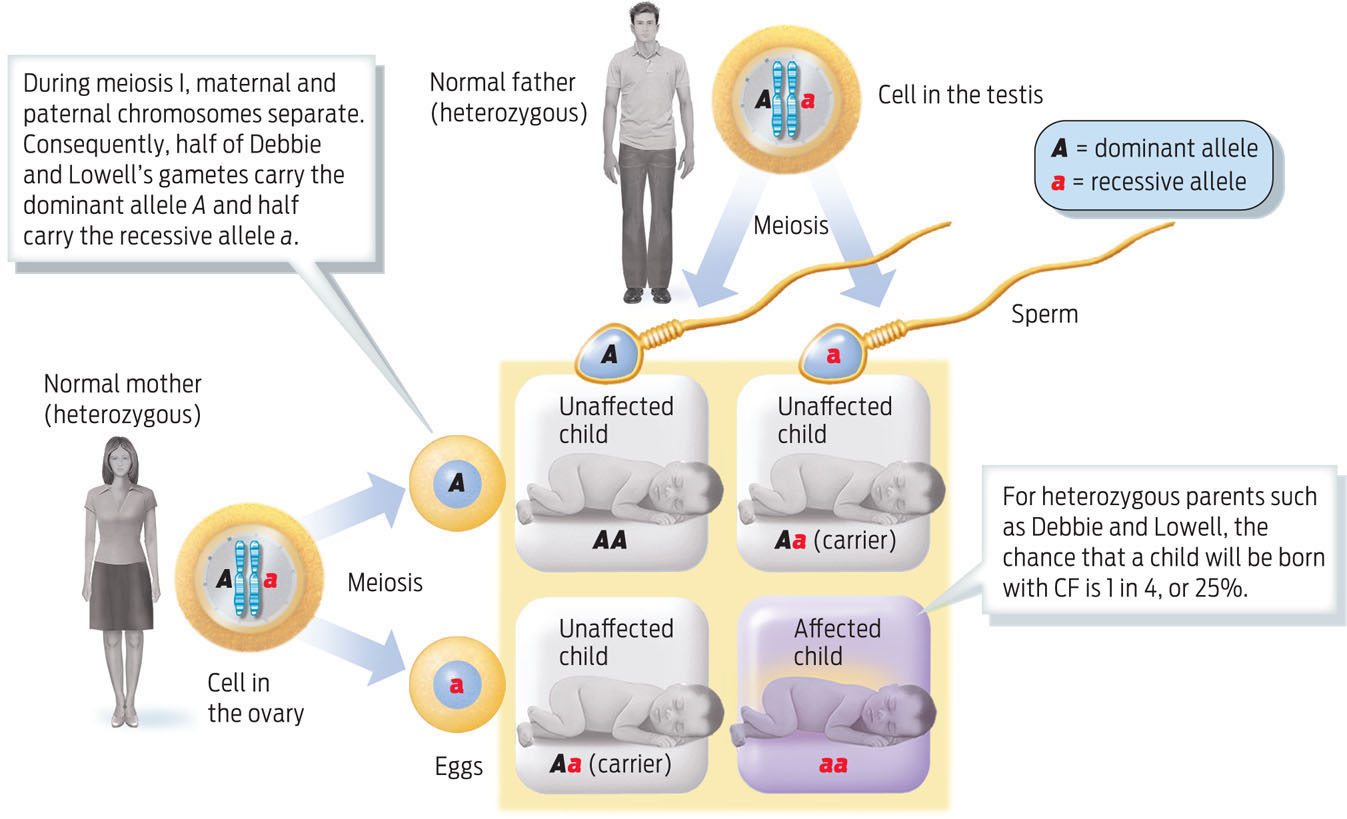
239
Just as Emily’s genotype is different from her parents’ genotype, Emily’s children will have different genotypes from her own. Whether or not her children develop CF depends on the father’s genotype. Since Emily is homozygous, she can contribute only recessive CF alleles to her children. If Emily were to have children with a man who had two normal alleles, for example, none of her children would have the disease—they would all have a heterozygous genotype but a normal phenotype. But as carriers they could pass on the disease to their children. If Emily had children with a man who was heterozygous for the CF gene, then her children would have a 1 in 2, or 50%, chance of having CF.
Not all recessive alleles cause disease. Many physical traits are the result of inheriting two recessive alleles of a gene. For example, people with blue eyes or red hair have inherited recessive alleles that prevent the deposition of dark pigment. And not all genetic diseases are caused by recessive alleles: some, such as the neurodegenerative disorder Huntington disease, are determined by dominant alleles. Diseases caused by dominant alleles have a higher probability of showing up in the next generation because it takes only one disease allele to cause the trait (INFOGRAPHIC 11.8).
Some genetic conditions, such as Huntington disease, a degenerative neurological disease, and polydactyly, having more than five fingers or toes per limb, are caused by dominant alleles. Many common traits such as dark eyes and dimples are also determined by dominant alleles. In these cases, inheriting one copy of the dominant allele is sufficient to display the trait.
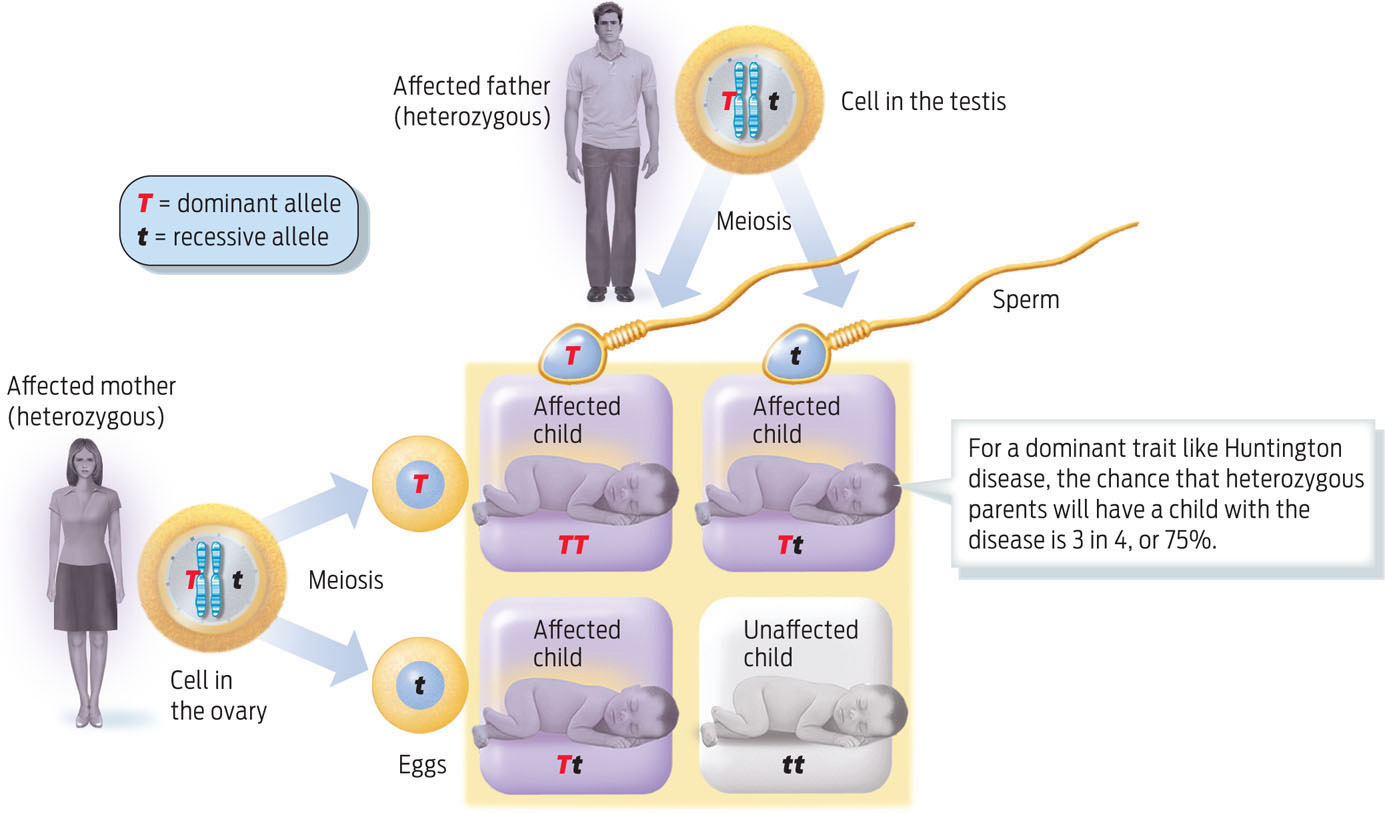
In all cases, anyone with a genetic disease is at risk for passing it on to his or her children. The risk merely varies, depending on whether the alleles are dominant or recessive, and on the genotype of the partner (TABLE 11.1).
| RECESSIVE TRAIT | PHENOTYPE |
|---|---|
| Albinism | Lack of pigment in skin, hair, and eyes |
| Cystic fibrosis | Excess mucus in lungs, digestive tract, and liver; increased susceptibility to infections |
| Sickle-cell disease | sickled red blood cells; damage to tissues |
| Tay-Sachs disease | Lipid accumulation in brain cells; mental deficiency, blindness, and death in childhood |
| DOMINANT TRAIT | PHENOTYPE |
| Huntington disease | mental deterioration and uncontrollable movements; onset at middle age |
| Freckles | Pigmented spots on skin, particularly on face and arms |
| Polydactyly | more than five digits on hands or feet |
| Dimples | Indentations in the skin of the cheeks |
| Chin cleft | Indentation in chin |
240
Couples who carry disease genes needn’t feel that having children is a roll of the dice, however. There are ways to ensure that their children won’t develop the diseases they could otherwise inherit. Many couples in this situation use a technology called pre-implantation genetic diagnosis to detect and select embryos that do not carry defective alleles. Through in vitro fertilization, a man’s sperm can fertilize a woman’s eggs outside the body (see Chapter 28). The genes of each resulting embryo are then examined for specific alleles, and then only embryos that don’t contain defective alleles are implanted into the mother. Hundreds of thousands of babies have been born by this technique. Some couples, however, may choose not to undergo assisted reproduction because of religious or other personal reasons.
Couples who carry disease genes needn’t feel that having children is a roll of the dice.