GEOLOGY
 How old is Earth, and how do we know?
How old is Earth, and how do we know?
ANSWER: When the Apollo 11 astronauts Neil Armstrong and Buzz Aldrin returned to Earth from their historic 1969 moon walk, they carried with them a cargo of lunar rock chipped from the moon’s surface. Embedded within these hunks of shimmering anorthosite lay clues to the earliest history of our solar system, including the planet we call home.
According to the nebular hypothesis, the most widely accepted explanation for the formation of our solar system, the planetary objects in our solar system are the result of a single event: the collapse of a swirling solar nebula, which formed both the sun and the planets out of cosmic dust. Since all the planets were formed at roughly the same time, we can date the age of the solar system by dating any planetary object within it. Of the many moon rocks obtained over the course of the six Apollo missions, the oldest have been calculated to be some 4.4 to 4.5 billion years old, which means that Earth is at least that old as well.
373
374

Why go to the moon to date Earth? With the exception of a few meteorite battle scars, the moon’s surface has remained largely intact over the course of its existence. In contrast, Earth is a swirling ball of molten lava that continuously churns and digests its rocky outer crust. Because of this perpetual changing, it is difficult to find original, undisturbed rocks from Earth’s earliest period. The oldest known rocks on Earth’s surface, the Acasta Gneiss in a remote region of northern Canada, are 4.0 billion years old. Other ancient Earth rocks, from Western Australia, contain single mineral crystals that are 4.4 billion years old.
RADIOMETRIC DATING The use of radioactive isotopes as a measure for determining the age of a rock or fossil.
While these values do not establish an absolute age of Earth, they do provide a lower limit: Earth is at least as old as the materials that make it up. From these Earth minerals and moon rocks, as well as material from meteorites that have fallen to Earth, scientists estimate that the age of Earth—and of the solar system more generally—is 4.54 billion years, give or take a few million years.
RADIOACTIVE ISOTOPES An unstable form of an element that decays into another element by radiation, that is, by emitting energetic particles.
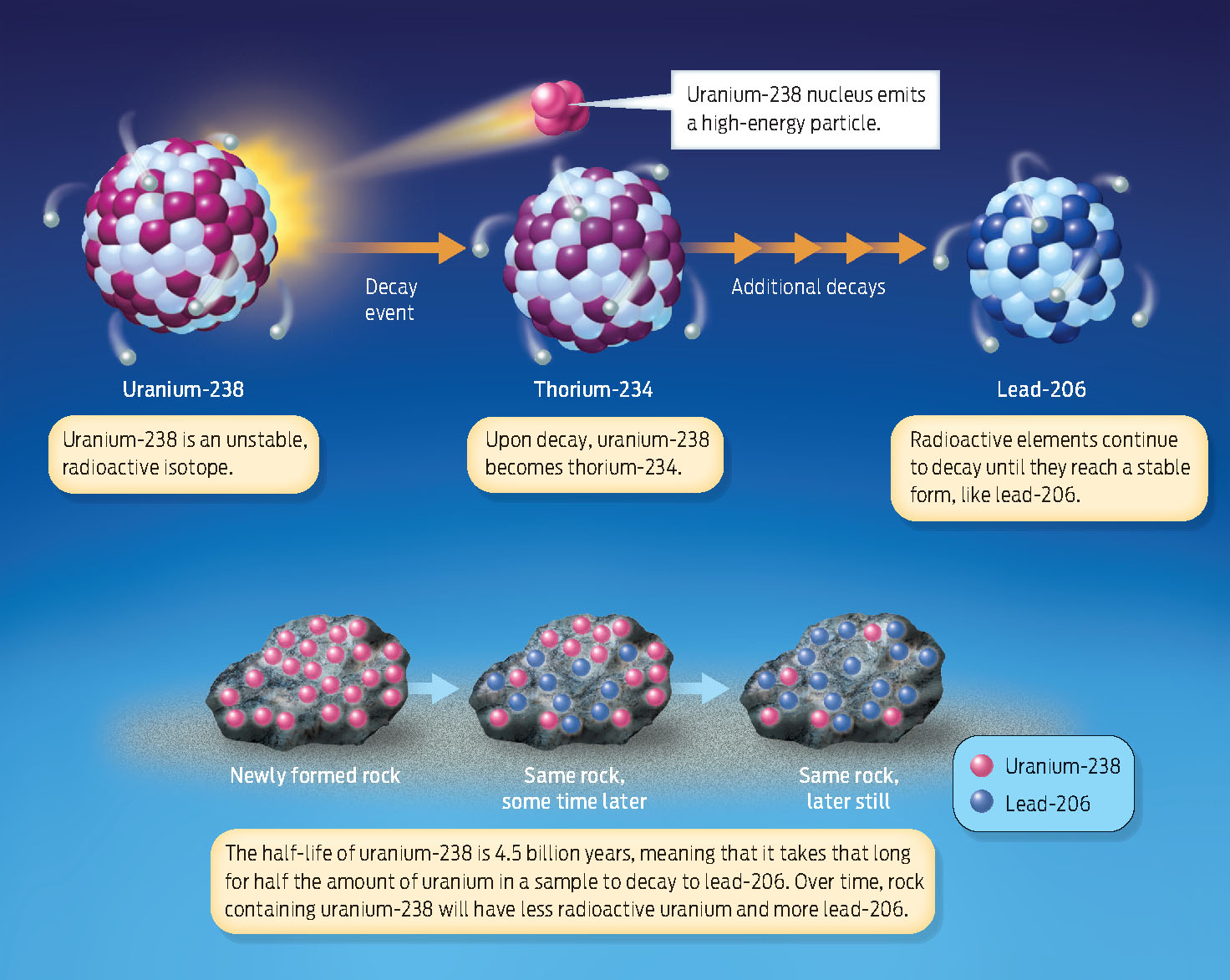
How are such rocks, extraterrestrial or earthly, dated? The most important method is radiometric dating, in which the amount of radioactivity present in a rock is used as a geologic clock. When rocks form, the minerals in them contain a certain amount of radioactive isotopes–atoms of elements such as uranium-238, potassium-40, and rubidium-87–that are unstable and decay into other atoms.
HALF-LIFE The time it takes for one-half of a sample of a radioactive isotope to decay.
Radioactive isotopes decay by releasing high-energy particles from the nucleus, a change that causes one element literally to transform into another. For example, an atom of the radioactive isotope uranium-238 eventually decays into a stable atom of lead-206. The time it takes for half the isotope in a sample to break down is called its half-life.
Different radioactive elements decay at different rates. Uranium-238 has a half-life of 4.5 billion years, whereas potassium-40 has a half-life of 1.3 billion years. The half-life of carbon-14 (useful for dating once-living, organic remains) is relatively short: it decays to nitrogen-14 in just 5,730 years. Because the isotopes decay at a known and constant rate, they can be used to determine the age of the materials in which they’re found (INFOGRAPHIC 17.1).
Radioactive isotopes are unstable versions of elements that undergo a process of radioactive decay, whereby they emit energy and are converted to another element.
As wind and water washed over rocks throughout Earth’s history, they stripped off, or eroded, particles and carried them to other places. Sometimes the deposited particles were compressed over many years into new rock layers by water or by additional particles. Such rock, called sedimentary rock, can be seen in the distinctive striations, or stripes, marking successive layers of sandstone and limestone found in former riverbanks like those surrounding the Grand Canyon in Arizona. Most fossils are found in sedimentary rocks.
Rocks can also form suddenly as erupting volcanoes spew lava and ash over an area. When this molten debris cools and hardens, it forms what is called igneous rock (“igneous” is from the Latin word for “fire”).
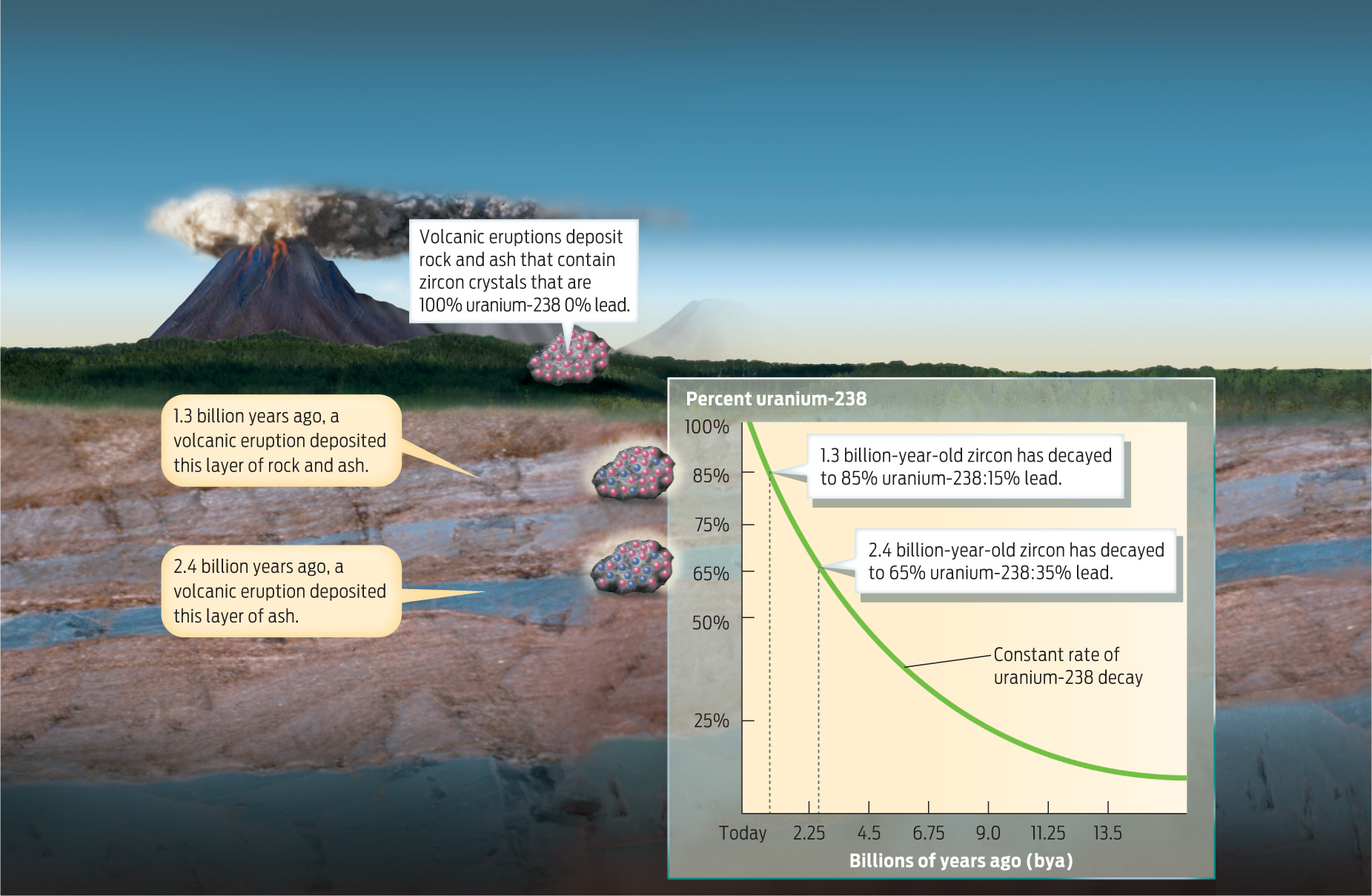
Radiometric dating is typically performed on igneous rocks, with the uranium-lead method used to date the oldest igneous rocks. Here’s how it works: When igneous rocks form, crystals of the mineral zircon are produced within the rock. Zircon crystals have a highly ordered structure that incorporates uranium but excludes lead; when these crystals first form, there is no lead present and the radioactive clock is set to zero. Over time, the uranium decays at a constant rate into lead-206. By measuring the ratio of uranium to lead in these crystals, scientists can calculate the age of the rock (INFOGRAPHIC 17.2).
Some rock types, like those produced during volcanic eruptions, contain radioactive minerals like zircon that can be used to determine the age of the rock. Because the isotope uranium-238 decays to lead at a constant rate, the age of rock layers containing these minerals can be calculated by measuring the ratio of uranium-238 to lead-206 in the mineral sample.
375
Sedimentary rocks cannot be dated by radiometric methods because they are made up of particles from rocks of various ages. But when fossils are found in sedimentary rock between layers of igneous rock, the dating of the igneous layers gives an accurate estimate of the age range of the fossils sandwiched in between.
Dating rocks by radioactive isotopes is quite precise and can be confirmed by crosschecking with different methods. For example, scientists used three different methods to date minerals taken from layers of rock in Saskatchewan, Canada: the potassium-argon method yielded an age of 72.5 million years; the uranium-lead method, an age of 72.4 million years; and the rubidium—strontium method, an age of 72.54 million years.
By radioactive dating, scientists have established that Earth is indeed quite old—old enough for evolution to have been acting for billions of years. They have also been able to establish precisely the dates of key events in the timeline of life on Earth as represented in the fossil record.
376