23.2 Nuclear power harnesses the heat released in nuclear reactions to produce electricity.
In some ways, electricity generated using nuclear energy is no different than other forms of thermoelectric power (those that use heat to produce electricity). Just like power plants that run on oil or coal, nuclear plants use heat to boil water and produce steam, which is then used to generate electricity. The difference, really, is in where that heat comes from; coal and oil plants create it by burning fossil fuels. At a nuclear power plant, heat is produced through a controlled nuclear reaction—usually a nuclear fission reaction.
418
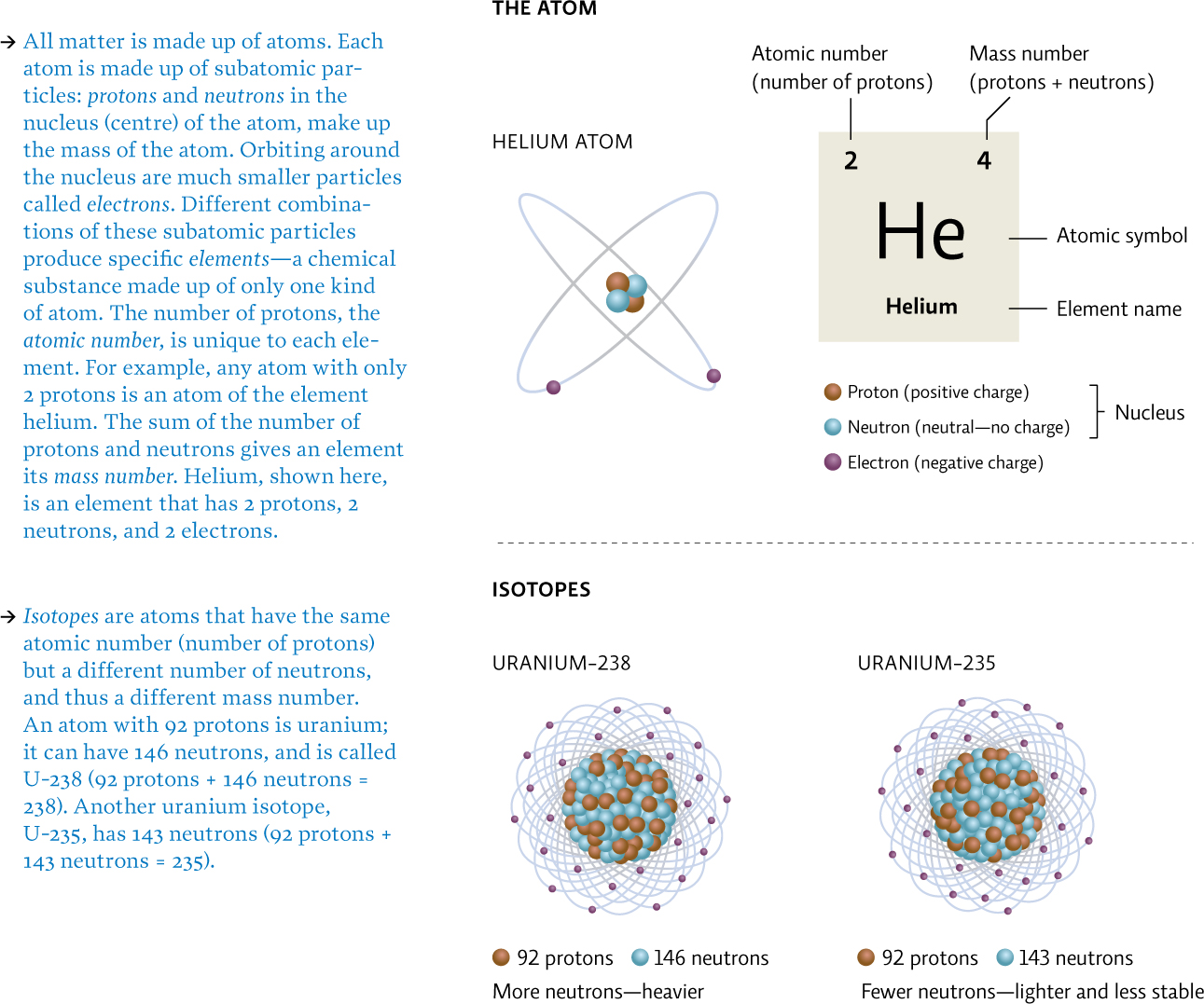
Nuclear fission reactions are those that result in the splitting of an atom. Their main ingredient is a special type of atom known as a radioactive isotope. Some elements can exist in two or more forms—each form has the same number of protons and electrons but a different number of neutrons, and hence, a different atomic mass; these different versions of the atom are called isotopes. [infographic 23.1]
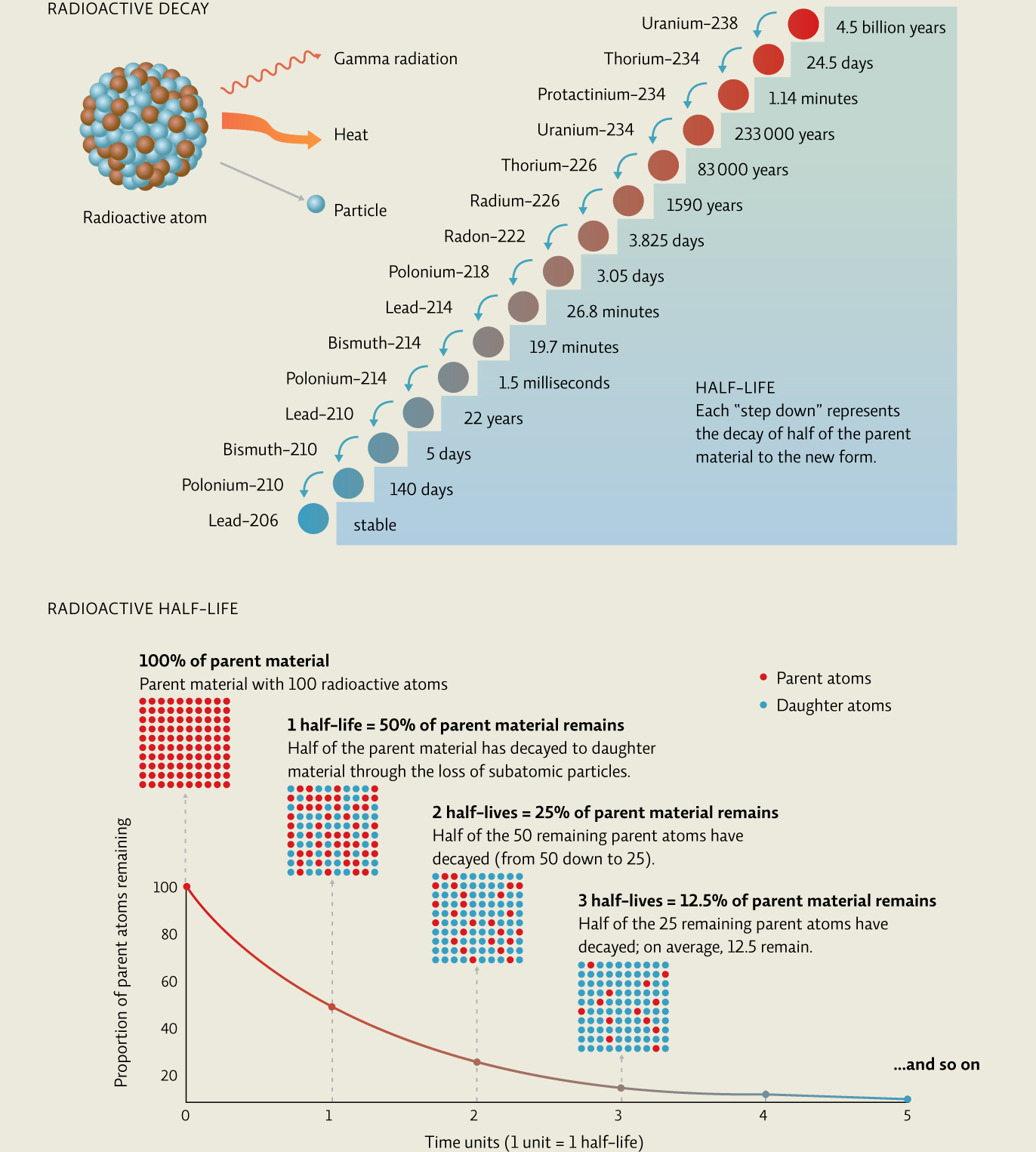
Most isotopes are stable, meaning they do not spontaneously lose protons or neutrons. But some are radioactive—they emit subatomic particles and heat energy (radiation) in a process known as radioactive decay. Radioactive decay is measured in half-lives. An isotope’s radioactive half-life is the amount of time it takes for half of the radioactive material in question to decay to a new form. So after one half-life, 50% of the material will decay; in the next half-life, 50% of what’s left (or 25% of the original amount) will then decay. After ten half-lives, just 0.1% of the original radioactive material is left. [infographic 23.2]
419
420
421
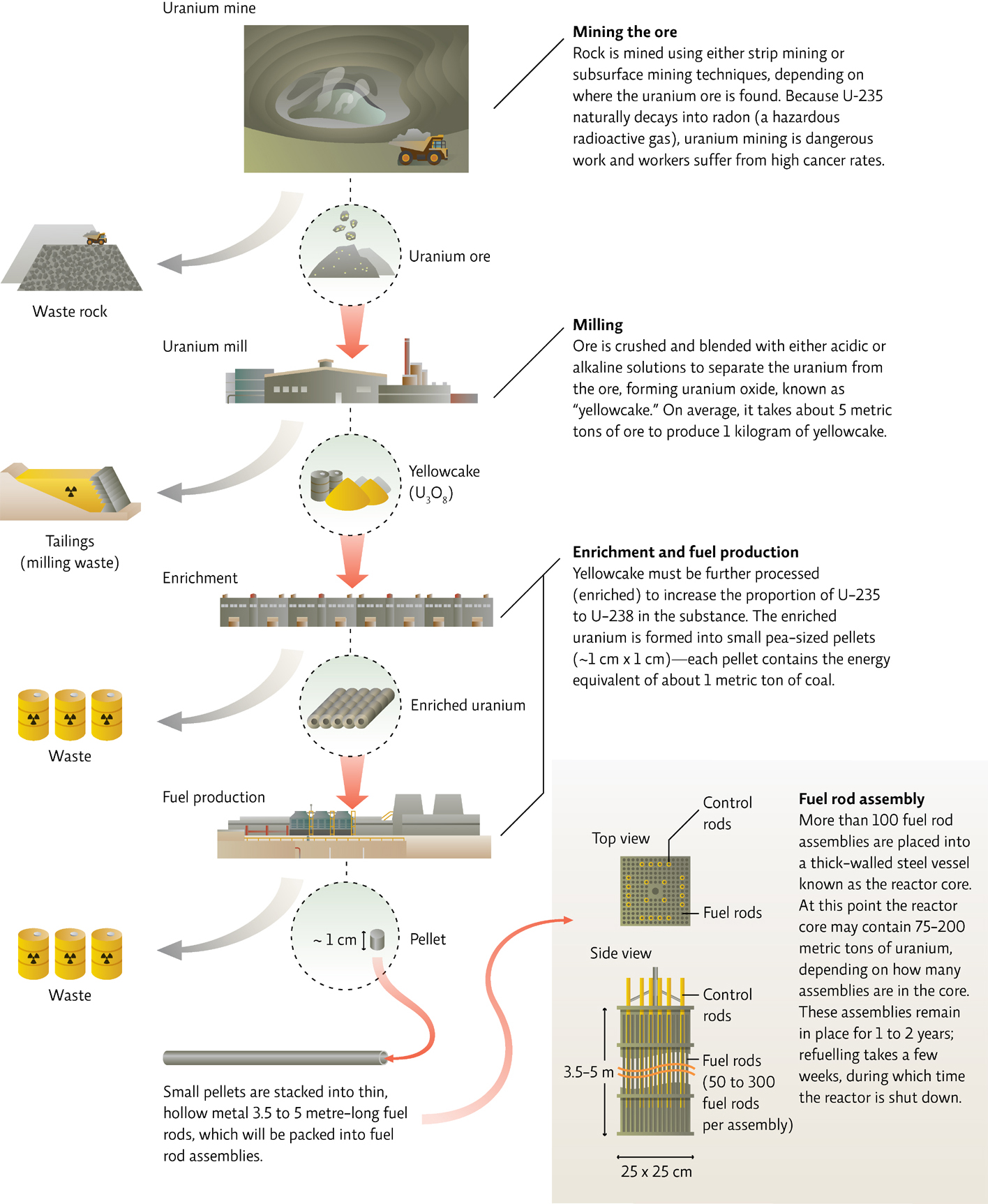
Most nuclear reactors use uranium, which has several isotopes. U-238 is the most stable and is the most abundant form of uranium—it makes up roughly 99% of Earth’s total supply. But it’s U-235, the most reactive form, that is mined from Earth, processed into nuclear fuel (which, like all mining work, involves both safety and environmental hazards), and packed into the fuel rods that are used in facilities like Fukushima. [infographic 23.3]
A nuclear fission chain reaction begins when U-235 in the fuel rods is deliberately bombarded with a neutron. This bombardment makes the nucleus unstable, causing it to split into a variety of two or more smaller atoms and releasing two or three additional neutrons in the process. These newly released neutrons then hit other U-235 atoms, causing them to split and release even more neutrons, and so on. [infographic 23.4]
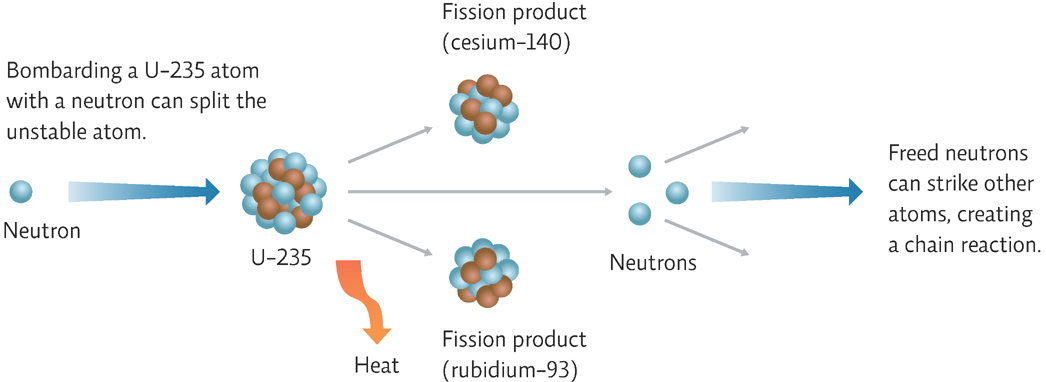
Unlike the type of nuclear reaction at work in a nuclear bomb, which uses much more radioactive material, setting off a chain reaction that is almost instantaneous, the reactions at nuclear power plants are highly controlled. Control rods—made of materials such as boron or graphite that absorb neutrons—are placed in the fuel rod assembly between the fuel rods to control the speed of the reaction. They can be added (to slow down or stop the reaction) or removed (to make it go faster).
Even controlled, this chain reaction releases a tremendous amount of heat—10 million times more than is released by burning fossil fuels like coal or oil. The heat is used to boil water, which produces steam, which turns turbines that create electricity.
All types of thermoelectric power take a lot of water—that’s why power plants are sited near rivers and oceans; but at the moment, nuclear power requires the most—on average, around 2500 litres per megawatt hour (MWh), compared with 1900 litres per MWh for coal, and 605 litres per MWh for natural gas. (A MWh is the production of 1 megawatt—1 million watts—over an hour’s time.) That means a typical 1000-MW nuclear reactor requires roughly 2 500 000 litres of water per minute to flow through the cooling system. Though most of this water (more than 95%) is returned to the source (river or ocean), there are still problems with the release of warmer-than-normal water back into the environment, as well as damage to aquatic life that gets trapped in or against intake filters.
The reason for all that water is simple: with nuclear energy, water is needed not only to produce steam but also to keep spent fuel rods cool and to prevent the reactor from overheating (remember, heat is produced from radioactive decay of fission by-products, which continues even after the reactor is shut down and fission stops; so spent fuel needs constant cooling). Without water to cool them, fuel rods can melt, releasing large amounts of radioactivity; the fuel rod metal casing can also get hot enough to react with steam in a way that produces highly explosive hydrogen gas.
422