2.2 Science gives us tools to observe the natural world.
Solomon and the team had decided to fly to the other end of the world after reading a scientific paper published the year before, in which Joe Farman of the British Antarctic Survey and his colleagues showed that, since the late 1970s, the ozone layer had thinned by about a third during the Antarctic spring.
The British team had collected nearly three decades of data in Antarctica, starting in 1957 with on-the-ground instruments. Like all good scientists, Farman and his team depended on observations (information detected with the senses or with equipment that extends our senses) of the natural world. His team collected data on the atmosphere’s composition (lower than normal ozone levels) and then used these observations to draw conclusions or make inferences—explanations of what else might be true or what might have caused the observed phenomenon.
23

Farman’s group also connected their results to studies by other researchers that had shown higher concentrations of an important compound: chlorofluorocarbons (CFCs), which in turn produce atmospheric chlorine (Cl). The concentrations of these chemicals seemed to increase at a rate matching the disappearance of ozone. The observation of a decrease in ozone did not come from just a few readings, but represented data collected at two different sites over more than a dozen seasons. Replication within a study (multiple test subjects or measurements) and between studies (independent tests that collect the same data, preferably conducted by other researchers) increase the reliability of the data. When replicates produce similar results it is less likely that the original data was “a fluke” or an unusual response. Farman’s research exemplified this hallmark of good science—in this case multiple data points at two different testing sites. His team’s results have subsequently been confirmed by other researchers.
Farman’s team inferred that the ozone depletion in the Antarctic was somehow connected to the increased presence of chlorine compounds in the atmosphere.
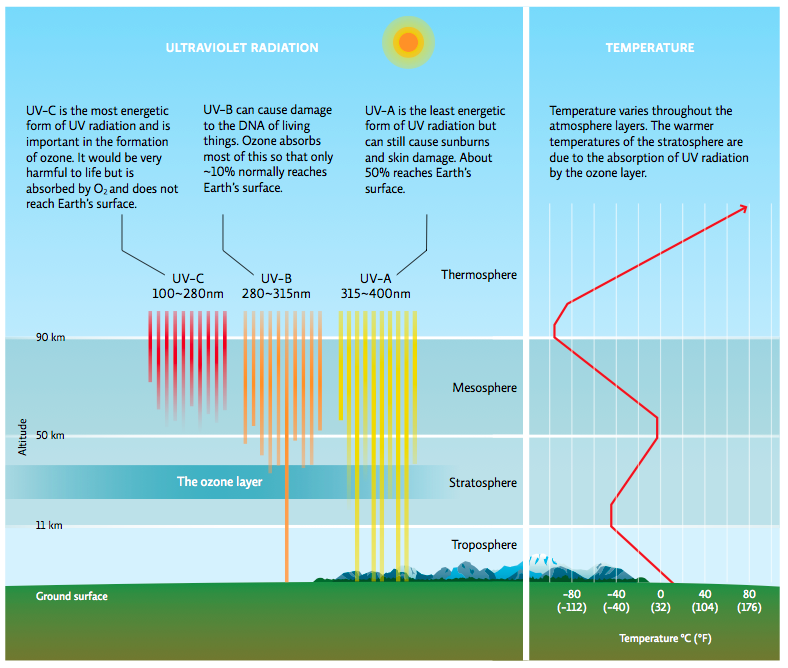
It was a serious proposition: without ozone, the world as we know it would not exist. Ozone is a key element of the atmosphere, the blanket of gases surrounding our planet that is made up of discernable layers, which differ in temperature, density, and gas composition. The lowest level, the troposphere, extends about 11 kilometres up. This level is familiar to us—it is the air we breathe and where our weather occurs. The next level in the atmospheric blanket, the stratosphere, rises about 50 kilometres above Earth’s surface. The stratosphere is much less dense than the troposphere but contains a “layer” of ozone (abbreviated as O3, because it contains 3 oxygen atoms), a region where most of the atmosphere’s ozone is found.

24
Without ozone, the world as we know it would not exist.
Solar radiation enters the atmosphere every day, including three forms of ultraviolet (UV) radiation: UV-A, UV-B, and UV-C. Ozone molecules in the stratosphere absorb much of the UV-B, a vital service since UV-B can damage cells and biological molecules like DNA. In humans, exposure to UV-B radiation increases the risk of cataracts, skin damage, and cancer. Fortunately, ozone prevents most of the UV-B from reaching Earth’s surface, where it can harm organisms. This stratospheric (good) ozone should not be confused with ground-level (bad) ozone found in the troposphere. Ground-level ozone is a component of smog and is harmful to living things (see Chapter 21). [infographic 2.1]
Susan Solomon became a scientist because she was curious about the natural world around her. Science is both a body of knowledge (facts and explanations) and the process used to get that knowledge. Understanding the process is more important than the “facts,” since facts may change as more information is collected through the scientific process. This process is a powerful tool that allows us to gather evidence to test our ideas, and to evaluate the quality of that evidence.
25
Science, however, is limited to asking questions about the natural world—not all questions are open to science. Scientific investigation, in both the natural and social sciences, is based on data gathered through empirical evidence, or observations. Only physical phenomena that can be objectively observed—meaning data that could be collected by anyone in the same place, using the same equipment, etc.—are fair game for science. Scientists can gather empirical evidence about the environment and living things using a wide variety of tools, including natural tools, such as their eyes, ears, and other senses. Phenomena that are not objectively observable (What is my dog thinking? Do ghosts exist?) and ethical or religious questions (Is economic growth more important than environmental health? What is the meaning of life?) cannot be empirically studied, and therefore are not under the purview of science.
Arriving at McMurdo Station, Solomon knew she had to apply the scientific process to understand why the ozone layer was thinning in the Antarctic. And she already had a culprit in mind.