3.5 What are the dangers presented by toxic substances and how do we determine safe exposure levels?
Between 2007 and 2009, the Canadian government analyzed urine samples from a representative sample of Canadians, and found that most—91%—tested positive for BPA. These results roughly matched what other countries, such as the United States and Germany, had also found. Since there are no known natural sources of BPA, this study confirmed that the chemical was, in fact, leaching from food and beverage containers into our bodies.
But did that necessarily mean that BPA was causing problems?
Any given substance has several characteristics we must consider when evaluating its safety. One such characteristic is its persistence—the ability of a substance to remain in its original form, often expressed by a measure of how long it takes to break down in the environment. Chemicals with low persistence tend to break down quickly in the presence of sunlight. Chemicals with high persistence tend to linger for a long time and can affect ecosystems well after their initial release.
44

Another trait that must be considered is the substance’s solubility—its ability to dissolve in liquid or gas. In some cases, water-soluble chemicals are safe for humans but not so good for the environment. Because we can excrete them in our urine, they don’t linger in our bodies for very long (of course, at high enough doses these chemicals can still prove toxic; and at low but continual doses they can cause kidney damage). Because water-soluble chemicals are easily taken up by aquatic organisms, they can wreak slow havoc on aquatic environments, and by extension, on the ecosystems that surround them.
Fat-soluble chemicals, those that do not dissolve in water, present an extra level of complexity. Because these chemicals pass easily through cell membranes, our cells can readily absorb them. Our bodies have a hard time expelling fat-soluble chemicals once they’re inside us. In some cases, the liver can covert a fat-soluble molecule into a water-soluble one, so that it can be broken down and excreted in urine. But when our livers can’t work this magic, fat-soluble chemicals are stored in our fatty tissue, where they can pile up in a process known as bioaccumulation.
Biomagnification describes a consequence of bioaccumulation; it’s what happens when animals that are higher up on the food chain eat other animals that have bioaccumulated toxic compounds. These predators consume their prey’s entire lifetime toxic dose. Biomagnification means that animals higher on the food chain accumulate far more toxic substances than do those lower on the chain. The best example in our human diets is tuna or swordfish. These fish are large predators, and thus are high up on the ocean food chain. So when we eat them, we consume all the chemicals that they have picked up from preying on smaller fish. Mercury is of particular concern. [infographic 3.2]
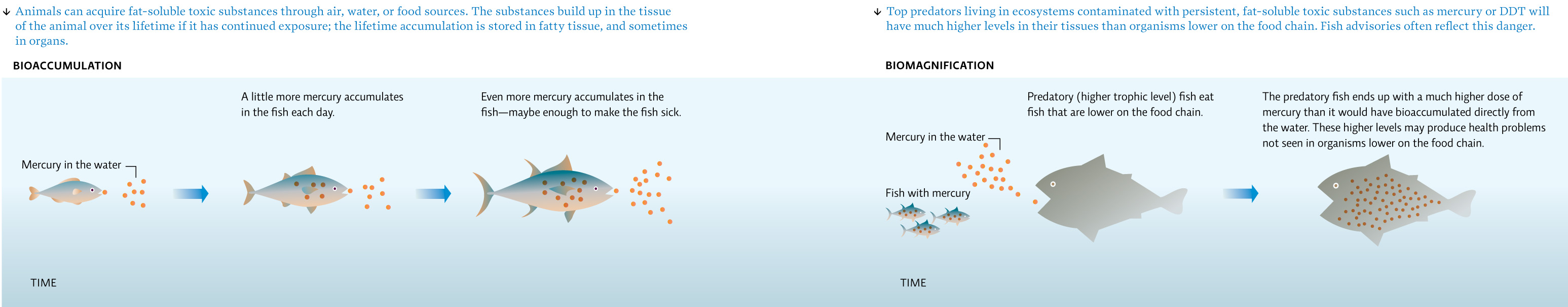
As for BPA, it has a low persistence, meaning that it breaks down rapidly in the environment. Although it is fat soluble, liver and gut cells can readily convert it to a water-soluble form, so that it’s easily excreted in urine. This means it should not bioaccumulate or biomagnify.
45
But BPA is so commonplace and people are exposed to it so continuously that it remains ever present in our systems, even if it isn’t accumulating; as we are breaking down and excreting some BPA, we are ingesting more. Scientists have spent the past decade trying to determine what such exposure might mean for human health.
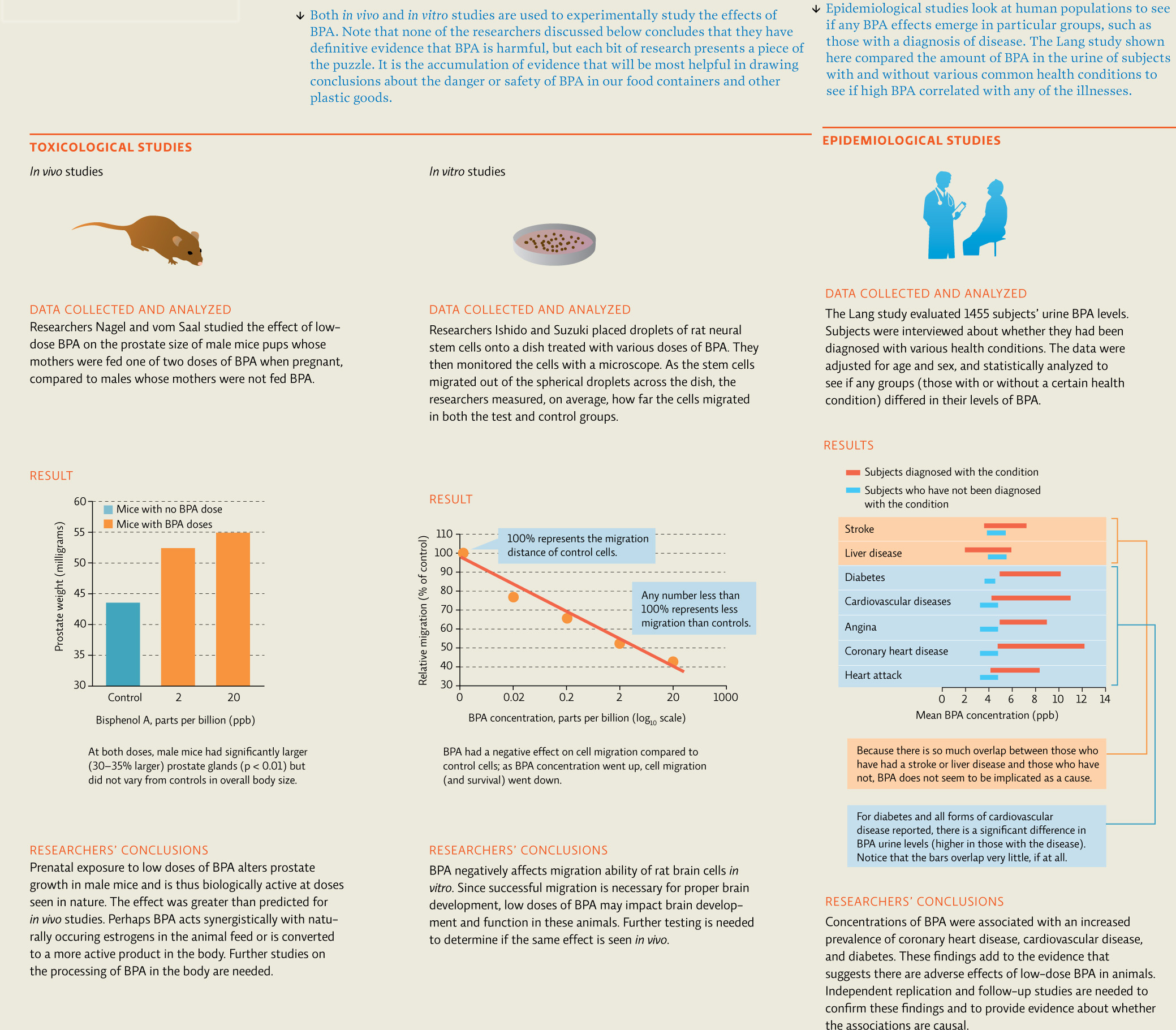
Figuring out the cause-and-effect relationships between our bodies and the chemicals that enter them is tricky work. Epidemiologists—the scientists charged with this type of research—can’t just expose a test group of humans to a toxic substance to see what effects it has. They must do a bit of detective work. They can start by looking for health problems in specific populations and work their way backward to find the culprit. Or they can look at groups that have been exposed to a given substance and see if any common health problems emerge. This latter approach is the one that researchers took for BPA. Epidemiologists looked at the health profiles of hundreds of individuals who had BPA in their urine; in one study of 1455 such people, they found an association between BPA concentrations and cardiovascular disease.
The task of determining exactly how BPA might go about wreaking such havoc inside human bodies falls to toxicologists. Toxicologists concern themselves with determining the specific properties of potentially toxic substances and how they affect cells or tissues. They do this by testing “intact” lab animals through in vivo (“in the body”) studies, or by testing cells in culture, such as in test tubes or Petri dishes, in what scientists call in vitro studies (“in glass”). Toxicologists use these data to determine how toxic a substance is and what its effects on living organisms are. [infographic 3.3]
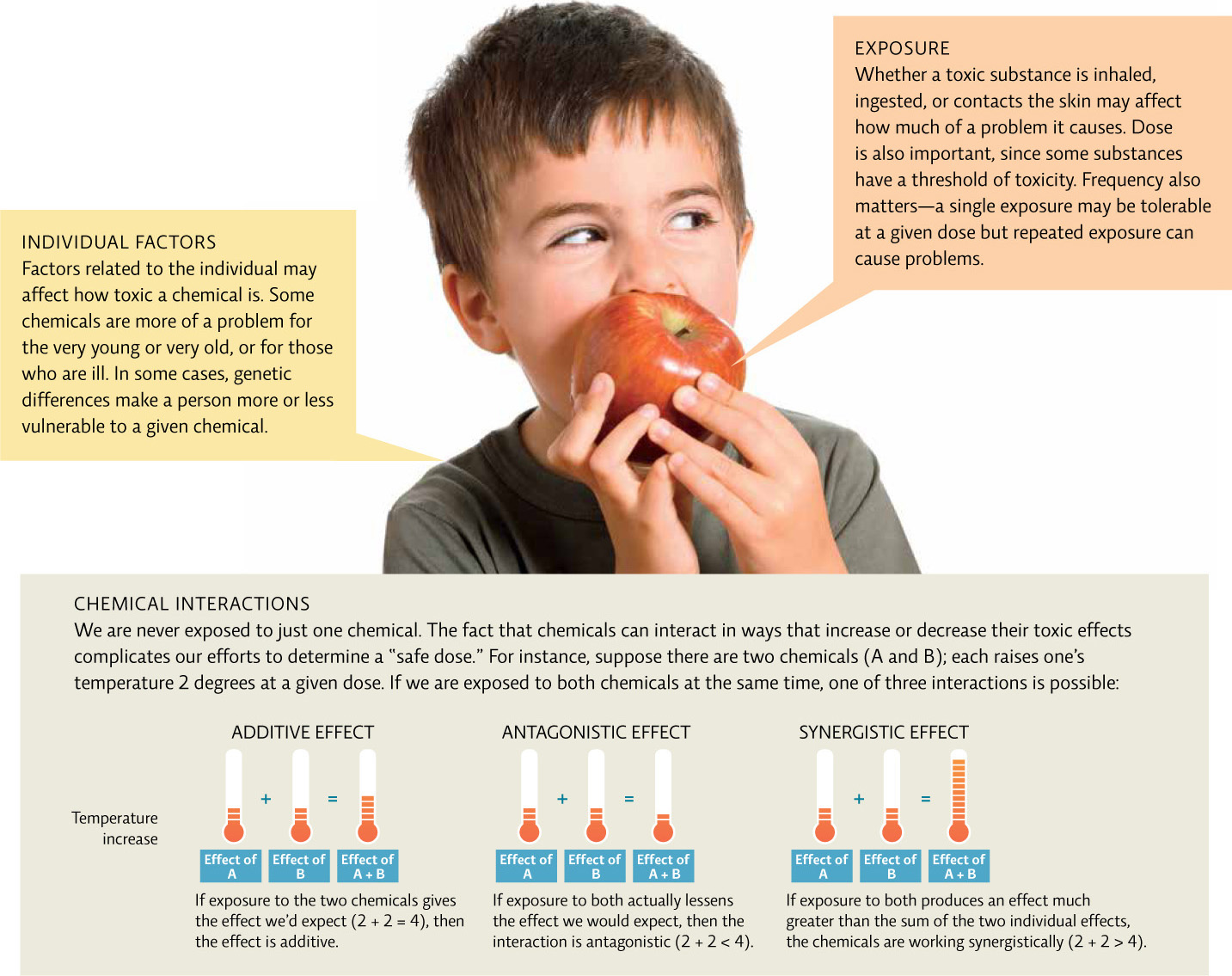
Toxicity can be affected by a host of factors. Individual susceptibility varies with genetics, age, and underlying health status. When a person is exposed to a toxic substance, the type and amount of chemicals already in that person’s system are important. Route of exposure (for example, inhalation, injection, or skin contact) and the dose at the time of exposure also play a role; large doses can cause immediate effects that differ from those caused by lower doses acquired over a longer time period. Some chemicals in the body might increase overall toxicity (additive effects); other chemicals may reduce toxicity due to interactions between the toxic substances that “cancel each other out” or at least lessen the effect (antagonistic effects). Still other chemicals may work together to produce an even bigger effect than expected (synergistic effects). [infographic 3.4]
46
47
But in general, toxicologists like to say that “the dose makes the poison.” This means that almost anything can be tolerated in low enough doses; conversely, anything—even water—can be toxic if the dose is big enough. And in most cases, as the dose increases, so does the severity of the effect. This idea—that higher doses of something harmful are worse for you than lower doses—makes obvious sense. It guides both regulatory efforts and modern medicine. And it applies to many other chemicals you can think of—except for the class of which BPA is a part: endocrine disruptors.