2-4 An object’s chemical composition is revealed by the unique pattern of its spectrum of light
The development of strategies to measure the temperature of a star so distant that we will probably never visit is exciting science in and of itself, but you might be wondering what these stars, galaxies, and giant interstellar clouds actually are. The astronomer and science popularizer Carl Sagan once quipped, “We are all star stuff,” but what does that actually mean? How might we determine what star stuff really is?
One strategy would be to send a space probe to the stars and do a chemical analysis. Unfortunately, the next star to our solar system is trillions of miles (trillions of kilometers) away, too far to send a probe. And even if we could send a space probe to a star, it is impossible to get close to stars because, like our Sun, they are far too hot. Fortunately, just as light gives us the clues to understand temperature, it gives us clues to chemical composition and what sorts of atoms these distant objects are made of. However, to figure this out, we have to exploit what we now understand about the structure of atoms. Why do different atoms each emit their own distinctive wavelengths of light? Why is a particular atom only able to absorb specific wavelengths of light? Answers to these questions did not become clear until astronomers began to use their knowledge of the structure and properties of atoms.
45
Electrons Can “Jump” Only to Specific Orbits Within Atoms, Creating the Particular Sets of Spectral Lines
You might be surprised to learn that only about 100 years ago, the inner workings of the atom were mostly unknown. Today, we understand that most of an atom’s mass is located in its very center. Tiny electrons, nearly without mass, are found in the outer extremities, often called electron orbits, while the majority of the atom’s volume is empty space. We know that the central mass of an atom contains two types of particles, protons and neutrons, but it is the behavior of the electrons in the outer extremities of the atom that generates light.
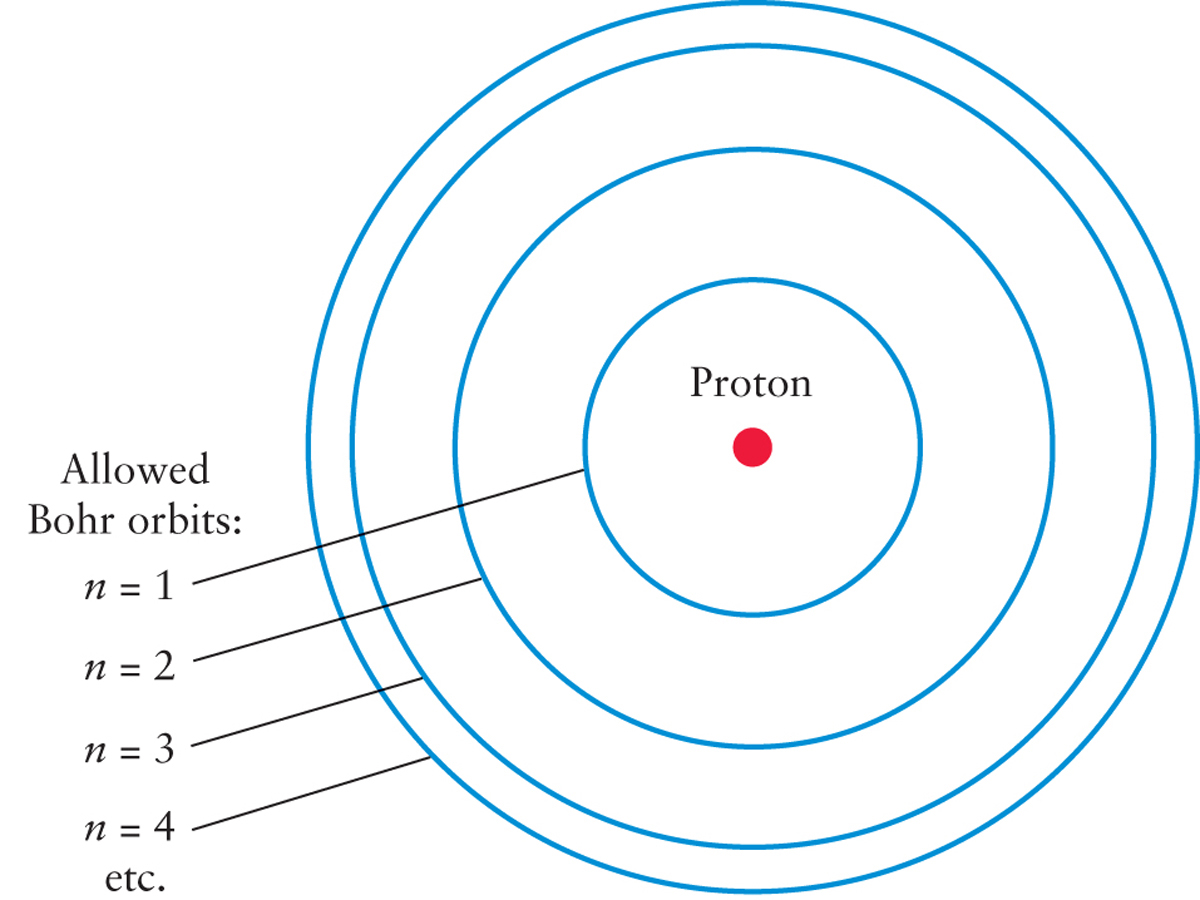
In the early 1900s, physicist Niels Bohr made the rather wild assumption that the electron in a hydrogen atom can orbit the nucleus only in certain specific orbits. (This was a significant break with the ideas of Newton, who predicted that any orbital path should be possible.) To be clear, the electrons in an atom do not actually move precisely in circular orbits, but thinking of it this way is an extremely handy conceptual tool.
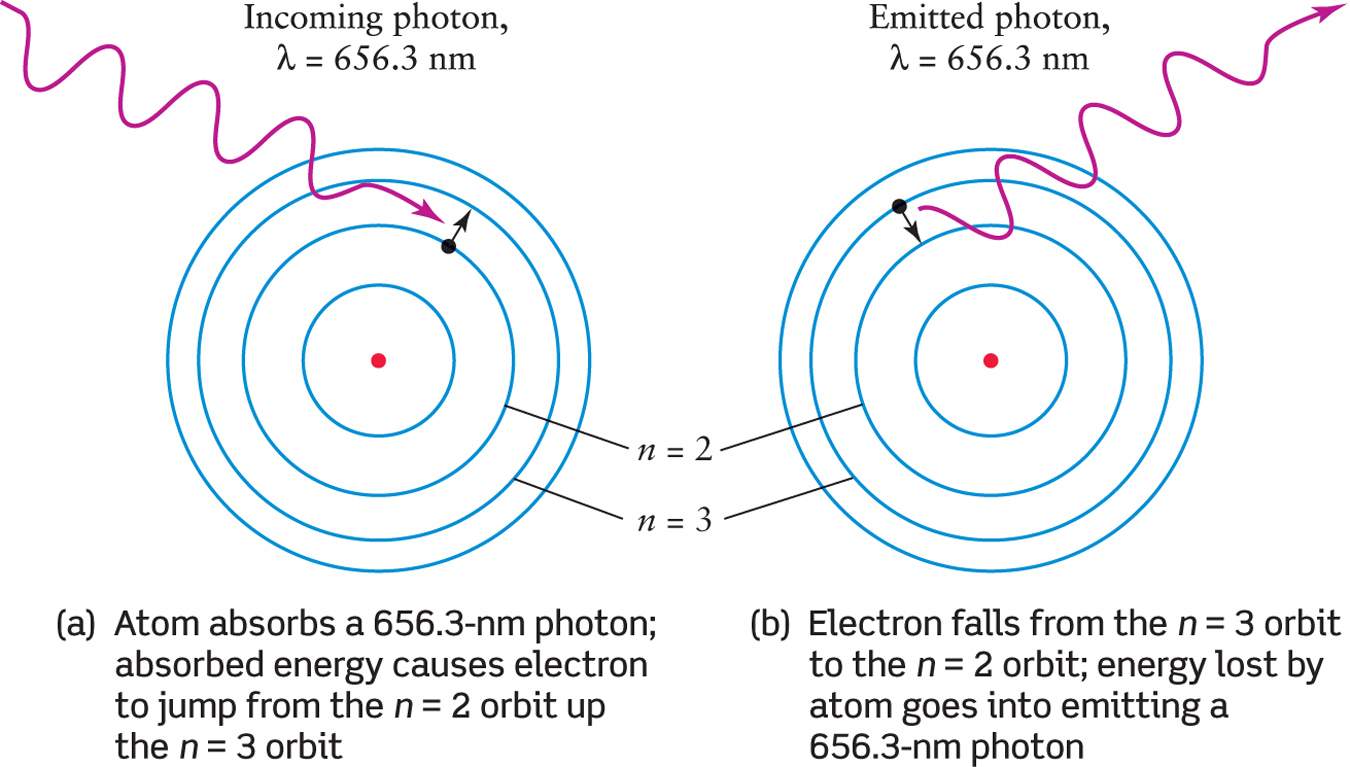
Figure 2-12 shows a hydrogen atom as an example of this mental model of an atom. The four smallest orbital paths are labeled by the numbers n = 1, n = 2, n = 3, and so on. In this example, an electron can jump from one of these orbits to another. For an electron to do this, the hydrogen atom must gain or lose a specific amount of energy. Figure 2-13 shows an electron jumping between the n = 2 and n = 3 orbits of the hydrogen atom as the atom absorbs or emits light. (In the case illustrated in Figure 2-13, the particular wavelength of light is 656.3 nm.) The atom must absorb energy for the electron to go from an inner to an outer orbit (Figure 2-13a); the atom must release energy for the electron to go from an outer to an inner orbit (Figure 2-13b). It is these transitions between electron orbit positions that help to explain the various spectra astronomers observe and to answer our key questions: Why do different atoms each emit their own distinctive wavelengths of light? Why is a particular atom only able to absorb specific wavelengths of light? The most important idea here is that an atom will only absorb or emit light if it has the precisely matched wavelength necessary to move the electron to a new orbit. There are two consequences to this notion. First, in most circumstances, only light with particular wavelengths will be emitted from an atom. Second, when light hits an atom, only particular wavelengths of light can be absorbed by an atom, while other wavelengths of light will pass through without affecting the atom.
46
Kirchhoff’s Laws Explain How Different Types of Spectra Are Produced
Once the nature of the atom was understood, astronomers were able to generalize three important statements about the nature of how spectra are produced, applying principles outlined years before by Gustav Kirchhoff, called Kirchhoff’s laws. These laws, which are illustrated in Figure 2-14, are as follows:
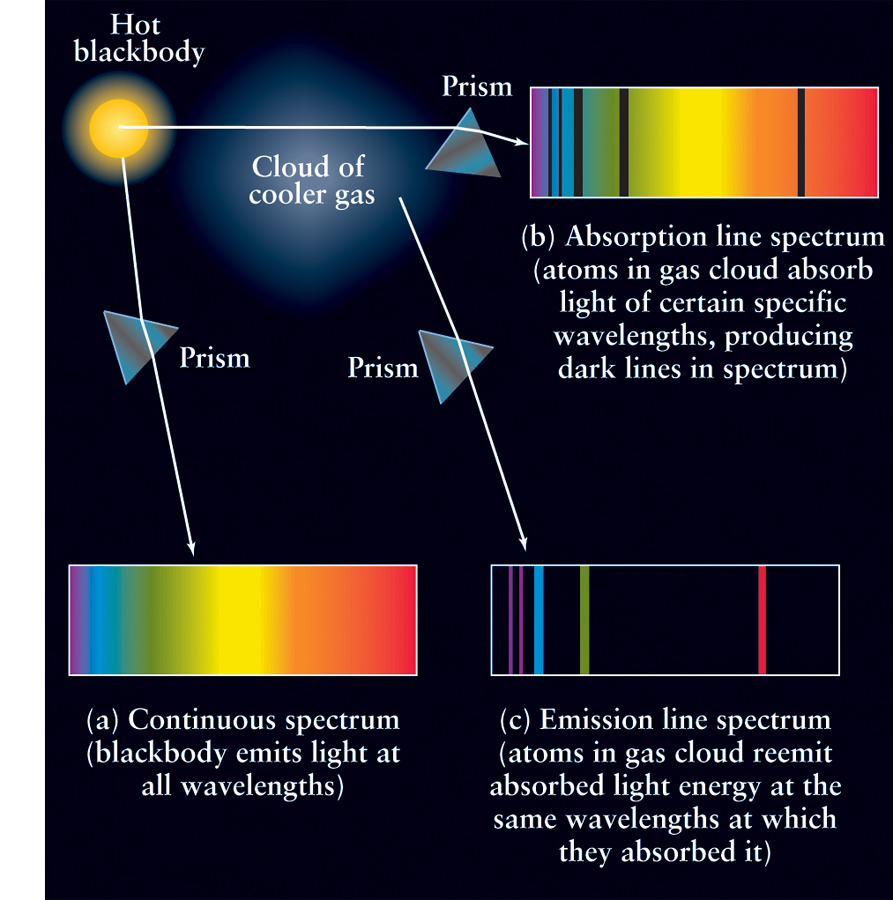
Law 1 A hot, opaque body or a hot, dense gas produces a continuous spectrum—a complete rainbow of colors without any spectral lines.
Law 2 A hot, transparent gas produces an emission line spectrum—a series of bright spectral lines against a dark background.
Law 3 A cool, transparent gas in front of a source of a continuous spectrum produces an absorption line spectrum—a series of dark spectral lines among the colors of the continuous spectrum. Furthermore, the dark lines in the absorption spectrum of a particular gas occur at exactly the same wavelengths as the bright lines in the emission spectrum of that same gas.
Kirchhoff’s laws imply that if a beam of white light is passed through a gas, the atoms of the gas somehow extract light of very specific wavelengths from the white light. This is a direct consequence of the behavior of electrons in an atom, as described in the previous section. Hence, an observer who looks straight through the gas at the white-light source will receive light whose spectrum has dark absorption lines superimposed on the continuous spectrum of the white light (Figure 2-14b). The gas atoms then radiate light of precisely these same wavelengths in all directions. An observer at an oblique angle (that is, one who is not sighting directly through the cloud toward the glowing object) will receive only this light radiated by the gas cloud; the spectrum of this light is bright emission lines on a dark background (Figure 2-14c).
47
CAUTION
Figure 2-14 shows that light can either pass through a cloud of gas or be absorbed by the gas. But there is also a third possibility: The light can simply bounce off the atoms or molecules that make up the gas, a phenomenon called light scattering. In other words, photons passing through a gas cloud can miss the gas atoms altogether, be swallowed whole by the atoms (absorption), or bounce off the atoms like billiard balls colliding (scattering). Box 2-3 The Heavens on Earth: Light Scattering describes how light scattering explains the blue color of the sky and the red color of sunsets.
Whether an emission line spectrum or an absorption line spectrum is observed from a gas cloud depends on the relative temperatures of the gas cloud and its background. Absorption lines are seen if the background is hotter than the gas, and emission lines are seen if the background is cooler.
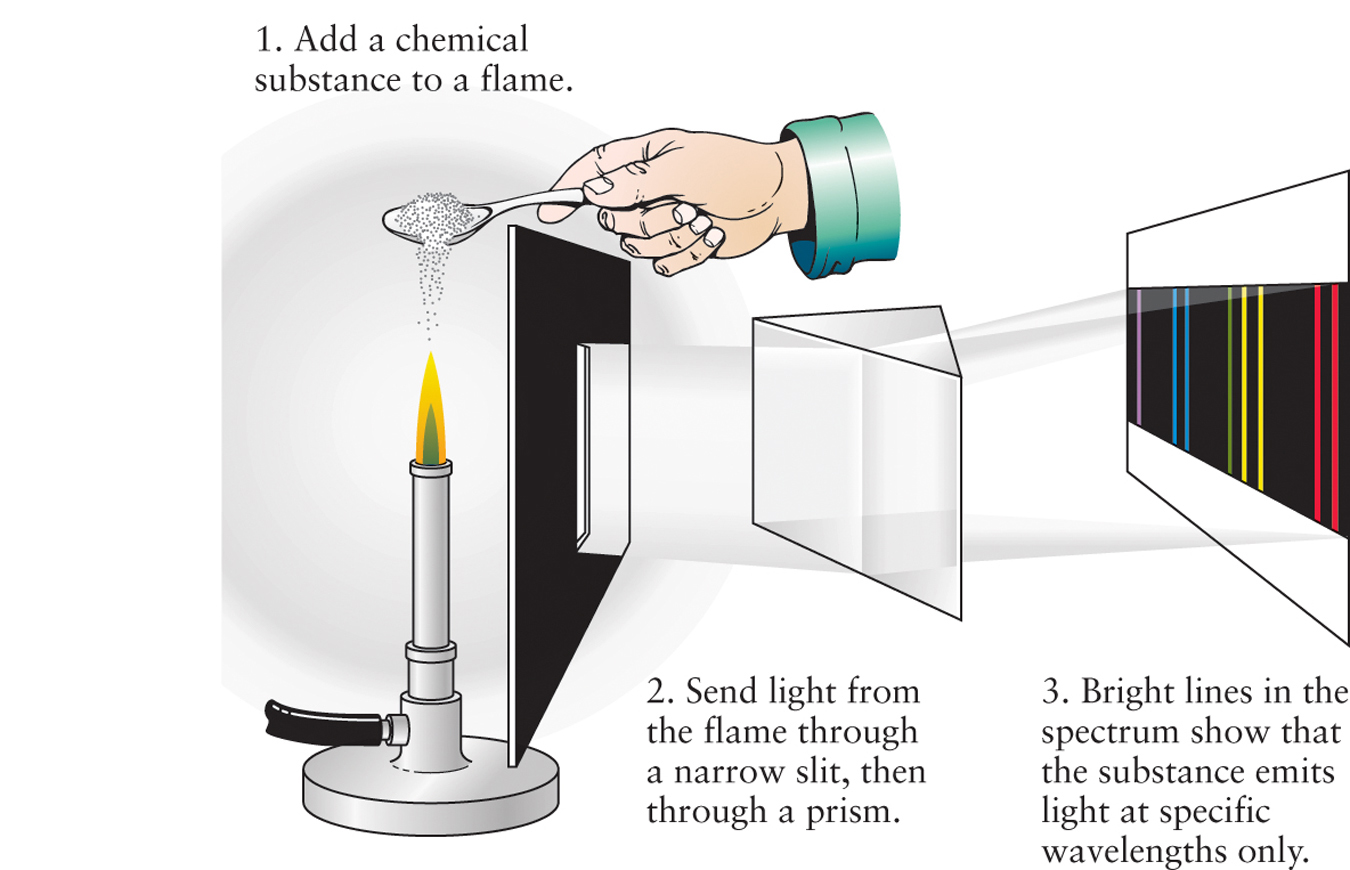
For example, if we drop sodium in the flame of a Bunsen burner in a darkened room, as illustrated in Figure 2-15, the flame will emit a characteristic orange-yellow glow. (This same glow is produced if we use ordinary table salt, which is a compound of sodium and chlorine.) If we look at the light from the flame through a prism, we observe an emission line spectrum with two closely spaced spectral lines at wavelengths of 588.99 nm and 589.59 nm in the orange-yellow part of the spectrum (see Figure 2-6).
What is truly remarkable about spectroscopy is that it can determine chemical composition at any distance. The 656-nm red light produced by a sample of heated hydrogen gas on Earth is the same as that observed coming from the glowing emission nebula in Figure 2-16, located about 170,000 light-years away.

The unique features of a spectrum can be deciphered to determine precisely which atoms make up the glowing object, even when it is very far way.
Liquids, Solids, and Dense Gases All can Produce Continuous Spectra
We have seen that changes to an electron’s orbit explain the emission line spectra and absorption line spectra of gases. But how can that explain the continuous spectra produced by dense objects like the filament of a lightbulb or the heated coils of a toaster? These objects are also made of atoms, so why don’t they emit light with an emission line spectrum characteristic of the particular atoms of which they are made?
The reason is directly related to the difference between a thin gas on the one hand and a dense gas on the other. In a thin gas, atoms are widely separated and can emit light without interference from other atoms. But in the case of a dense gas, atoms are so close that they almost touch, and thus these atoms interact strongly with each other. (In fact, a liquid and a solid can act like glowing, dense gas when heated to a high enough temperature.) These closely packed atom-to-atom interactions interfere with the process of emitting light. As a result, the pattern of distinctive bright spectral lines that the atoms would emit in isolation becomes “smeared out” into a continuous spectrum.
48
THE HEAVENS ON EARTH
Light Scattering
Light scattering is the process whereby photons bounce off particles in their path. These particles can be atoms, molecules, or clumps of molecules. You are reading these words using photons from the Sun or a lamp that bounced off the page—that is, were scattered by the particles that make up the page.
An important fact about light scattering is that very small particles—ones that are smaller than a wavelength of visible light—are quite effective at scattering short-wavelength photons of blue light, but less effective at scattering long-wavelength photons of red light. This fact explains a number of phenomena that you can see here on Earth.
The light that comes from the daytime sky is sunlight that has been scattered by the molecules that make up our atmosphere (see part (a) of Figure B2-3.1). Air molecules are less than 1 nm across, far smaller than the wavelength of visible light, so they scatter blue light more than red light—which is why the sky looks blue. Smoke particles are also quite small, which explains why the smoke from a cigarette or a fire has a bluish color.
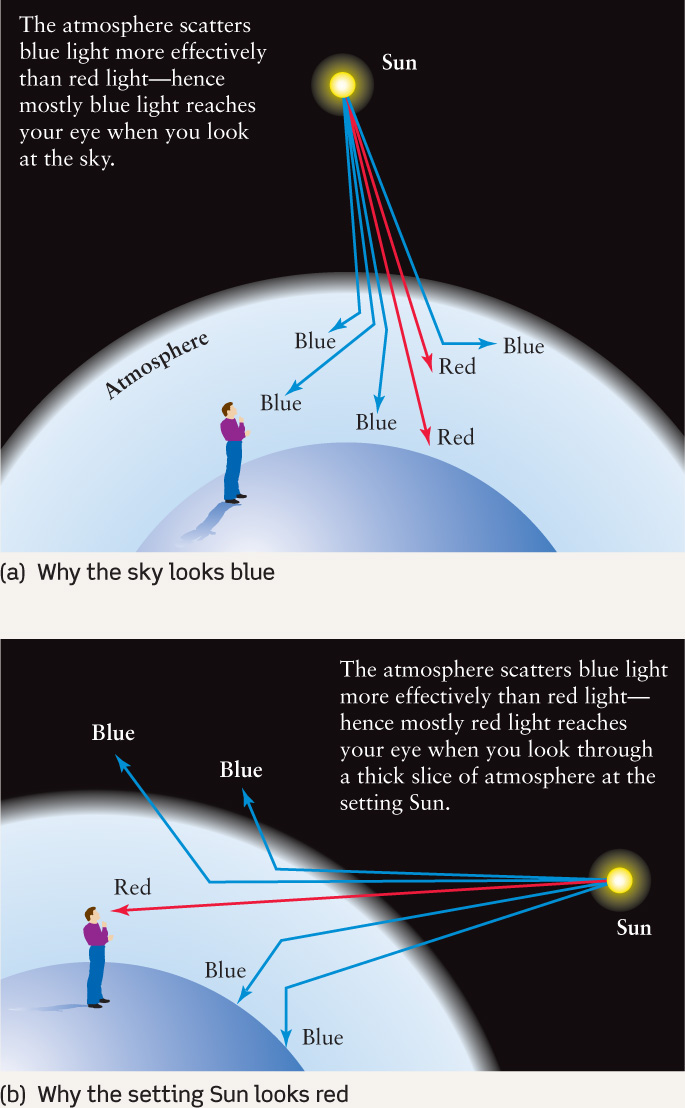
Distant mountains often appear blue thanks to sunlight being scattered from the atmosphere between the mountains and your eyes. (The Blue Ridge Mountains, which extend from Pennsylvania to Georgia, and Australia’s Blue Mountains derive their names from this effect.) Sunglasses often have a red or orange tint, which blocks out blue light. This cuts down on the amount of scattered light from the sky reaching your eyes and allows you to see distant objects more clearly.
Light scattering also explains why sunsets are red. The light from the Sun contains photons of all visible wavelengths, but as this light passes through our atmosphere the blue photons are scattered away from the straight-line path from the Sun to your eyes. Red photons undergo relatively little scattering, so the Sun always looks a bit redder than it really is. When you look toward the setting sun, the sunlight that reaches your eyes has had to pass through a relatively thick layer of atmosphere (part (b) of the figure). Hence, a large fraction of the blue light from the Sun has been scattered, and the Sun appears quite red.
The same effect also applies to sunrises, but sunrises seldom look as red as sunsets. The reason is that dust is lifted into the atmosphere during the day by the wind (which is typically stronger in the daytime than at night), and dust particles in the atmosphere help to scatter even more blue light.
If the small particles that scatter light are sufficiently concentrated, there will be almost as much scattering of red light as of blue light, and the scattered light will appear white. This explains the white color of clouds, fog, and haze, in which the scattering particles are ice crystals or water droplets. Whole milk looks white because of light scattering from tiny fat globules; nonfat milk has only a very few of these globules and so has a slight bluish cast.
Light scattering has many applications to astronomy. For example, it explains why very distant stars in our Galaxy appear surprisingly red. The reason is that there are tiny dust particles in the space between the stars, and this dust scatters blue photons. By studying how much scattering takes place, astronomers have learned about the tenuous material that fills interstellar space.
49
 Go to Video 2-2
Go to Video 2-2
Question
ConceptCheck 2-10: What type of spectra would result from a glowing field of hot, dense lava as viewed by an orbiting satellite through Earth’s atmosphere?
Each Chemical Substance Has a Unique Pattern of Spectral Lines
Now we are in a position to talk specifically about how atoms can be detected within and among the distant stars. You might have noticed that fireworks displays often show highly varied, glowing colors. What would you see if you could pass these colored lights through a prism? Actually, scientists have been studying the light from burning chemicals for more than a century. In the mid-1850s, the German chemist Robert Bunsen and the Prussian-born physicist Gustav Kirchhoff discovered that the light emitted from burning chemicals has a specific appearance when passed through a prism. In fact, every chemical substance has its own specific color spectrum when burned and passed through a prism, showing a pattern of thin, bright lines against a dark background known as spectral lines (Figure 2-16). The identification of chemical substances by the unique patterns of lines in their spectrum is called spectroscopy and is a core aspect of contemporary astronomy. Spectral lines are tremendously important in astronomy, because they provide reliable evidence about the chemical composition of distant objects. We will use them again and again throughout this book.