4-3 Spectroscopy reveals the chemical composition of the planets
We have seen that the average densities of the planets and moons give us a crude measure for what they are made of, or their chemical compositions. For example, the low average density of Earth’s Moon (3340 kg/m3) compared with Earth (5515 kg/m3) tells us that our Moon contains relatively little iron or other dense metals. But to truly understand the nature of the planets and moons, we need to know their chemical compositions in much greater detail than we can learn from average density alone.
The most accurate way to determine a planet’s chemical elements would be by directly analyzing samples taken from a planet’s atmosphere and soil. Unfortunately, of all the planets and moons, we have such direct information only for Earth and three other worlds on which spacecraft have landed—Venus, Moon, and Mars. However, as we saw in Chapter 2 (Section 2-4), astronomers can use spectroscopy to determine the compositions of even the farthest objects. Spectroscopy reveals the composition of planets both with and without a surrounding envelope of gas, known as the atmosphere.
Determining Composition: Planets with Surrounding Atmospheres
If a planet has an atmosphere, then sunlight reflected from that planet must have passed through parts of its atmosphere. During this passage, some of the wavelengths of sunlight will have been absorbed. Hence, the spectrum of this reflected sunlight will have dark absorption lines. Astronomers look at the particular wavelengths absorbed and the amount of light absorbed at those wavelengths. Both of these depend on the kinds of chemicals present in the planet’s atmosphere and the abundance of those chemicals. For example, Figure 4-3 shows how astronomers have used spectroscopy to analyze visible sunlight reflected from Titan, finding the presence of methane (CH4).
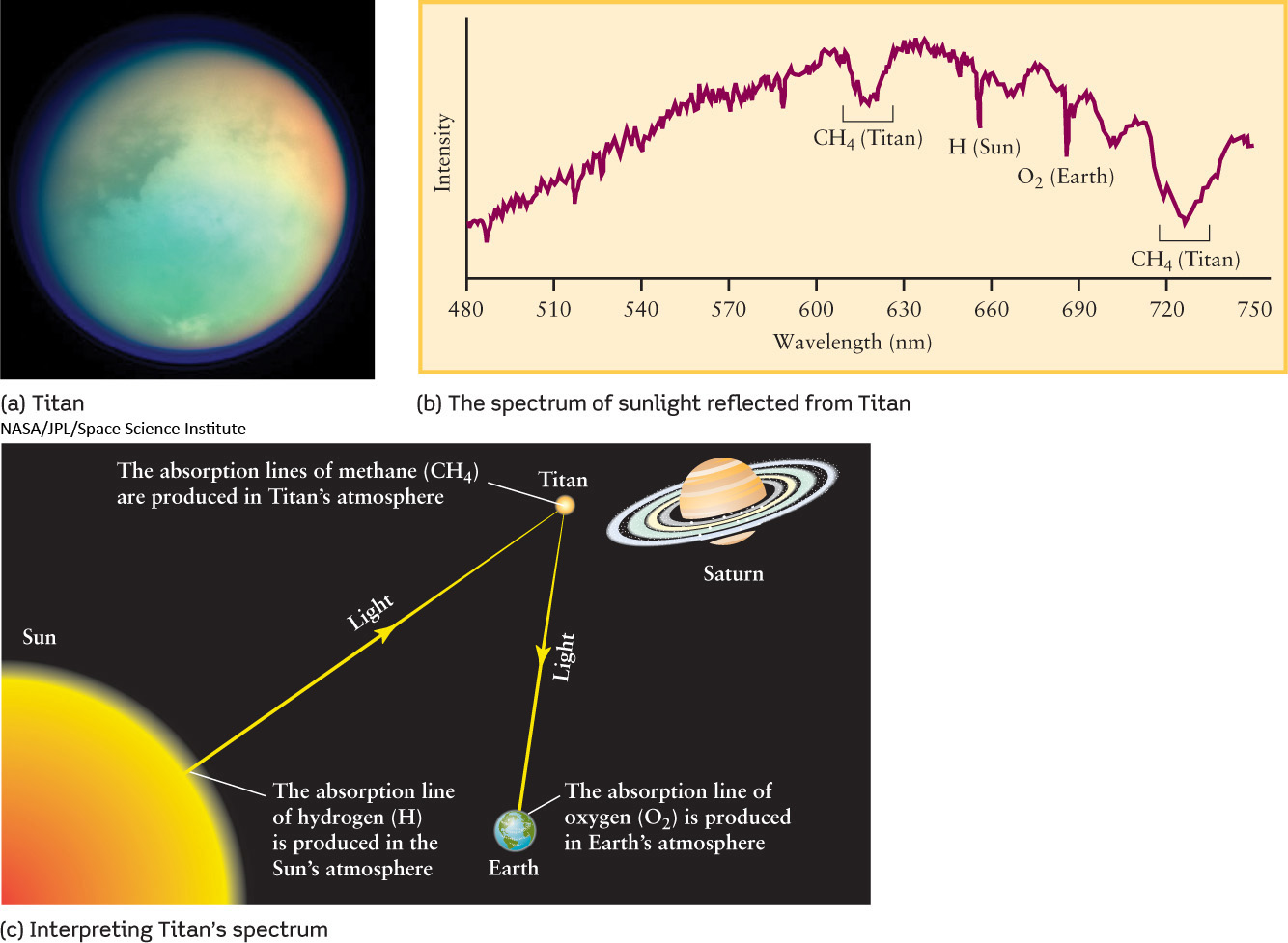
93
In addition to visible-light measurements, it is sometimes even more useful to study the infrared and ultraviolet spectra of planetary atmospheres. Many molecules exhibit much more obvious spectral lines in these nonvisible wavelength bands than in the visible regime. As an example, the ultraviolet spectrum of Titan shows that nitrogen molecules (N2) are the dominant constituent of Titan’s atmosphere. Furthermore, Titan’s infrared spectrum includes spectral lines of a variety of molecules that contain carbon and hydrogen, indicating that Titan’s atmosphere has a very complex chemistry. None of these molecules could have been detected by visible light alone. Since Earth’s atmosphere is largely opaque to infrared and ultraviolet wavelengths, telescopes in space are important tools for these spectroscopic studies of the solar system.
Question
ConceptCheck 4-5: If planets reflect the Sun’s light rather than emitting light of their own, how can spectroscopy reveal information about a planet’s atmosphere?
Determining Composition: Planets without Surrounding Atmospheres
Atmosphere is covered in detail in Chapters 6 and 7.
Spectroscopy can also provide useful information about the solid surfaces of planets and moons without atmospheres. When light shines on a solid surface, some wavelengths are absorbed while others are reflected. For example, a plant leaf on Earth absorbs red and violet light but reflects green light—which is why leaves look green. Unlike a gas, a solid object illuminated by sunlight does not produce sharp, definite spectral lines. Instead, only broad absorption features appear in the spectrum. By comparing such a spectrum with the spectra of samples of different substances on Earth, astronomers can infer the chemical composition of the surface of a planet or moon.
Planetary surfaces are covered in detail in Chapter 6.
As an example, Figure 4-4a shows Jupiter’s moon Europa, and Figure 4-4b shows the infrared spectrum of light reflected from the surface of Europa. Because this spectrum is so close to that of water ice—that is, frozen water—astronomers conclude that water ice is the dominant constituent of Europa’s surface.
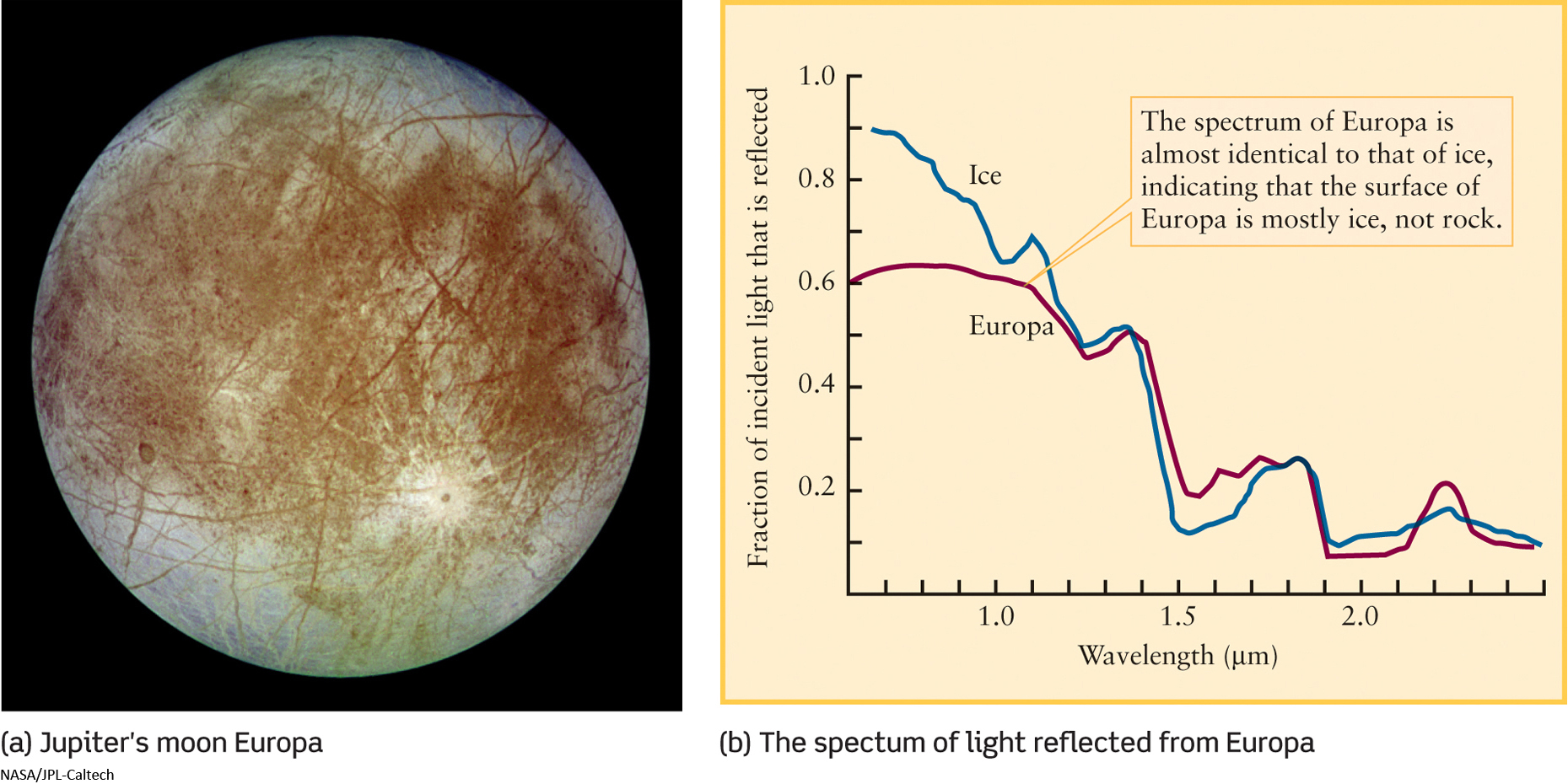
Unfortunately, spectroscopy tells us little about what the material is like just below the surface of a moon or planet. For this purpose, there is simply no substitute for sending a spacecraft to a planet and examining its surface directly.
94
Question
ConceptCheck 4-6: How is the spectrum of light observed from a solid planetary surface different from the spectrum of light observed from a gas composing an atmosphere?
The Jovian Planets Are Made of Lighter Elements than the Terrestrial Planets
Spectroscopic observations from Earth and spacecraft show that the outer layers of the Jovian planets are comprised primarily of the lightest gases, hydrogen and helium. In contrast, chemical analysis of solid samples from Venus, Earth, and Mars demonstrate that the terrestrial planets are made mostly of heavier elements, such as iron, oxygen, silicon, magnesium, nickel, and sulfur. Spacecraft images such as Figure 4-5 only hint at these striking differences in chemical composition.
Temperature plays a major role in determining whether the materials of which planets are made exist as solids, liquids, or gases. Hydrogen (H2) and helium (He) are gaseous except at extremely low temperatures and extraordinarily high pressures. By contrast, rock-forming substances such as iron and silicon are solids except at temperatures well above 1000 K. Between these two extremes are substances such as water (H2O), carbon dioxide (CO2), methane (CH4), and ammonia (NH3), which solidify at low temperatures (from below 100 K to 300 K) into solids called ices. (In astronomy, frozen water is just one of many kinds of “ice.”) At somewhat higher temperatures, they can exist as liquids or gases. For example, clouds of ammonia ice crystals are found in the cold upper atmosphere of Jupiter, but within Jupiter’s warmer interior, ammonia exists primarily as a liquid.
95
CAUTION
The Jovian planets are sometimes called “gas giants.” It is true that their primary constituents, including hydrogen, helium, ammonia, and methane, are gases under normal conditions on Earth. But in the interiors of these planets, pressures are so high that these substances are liquids, not gases. The Jovian planets might be better described as “liquid giants!”
As you might expect, a planet’s surface temperature is related to its distance from the Sun. The four inner planets are quite warm. For example, midday temperatures on Mercury may climb to 700 K (= 427°C = 801°F), and during midsummer on Mars, it is sometimes as warm as 290 K (= 17°C = 63°F). The outer planets, which receive much less solar radiation, are cooler. Typical temperatures range from about 125 K (= −148°C = −234°F) in Jupiter’s upper atmosphere to about 55 K (= −218°C = −360°F) at the tops of Neptune’s clouds.
How might a planet’s distance from the Sun, and its resulting surface temperature, explain the chemical elements we find on these planets? As it turns out, the atmospheres of the inner planets, whose orbits keep them close to the Sun, contain virtually no hydrogen molecules or helium atoms. Instead, the atmospheres of Venus, Earth, and Mars are composed of heavier molecules such as nitrogen (N2, 14 times more massive than a hydrogen molecule), oxygen (O2, 16 times more massive), and carbon dioxide (CO2, 22 times more massive). To understand the connection between surface temperature and the absence of hydrogen and helium, we need to know a few basic facts about gases.
The temperature of a gas is directly related to the speeds at which the atoms or molecules of the gas move: The higher the gas temperature, the greater the speed of its atoms or molecules. Furthermore, for a given temperature, lightweight atoms and molecules move more rapidly than heavy ones. On the four inner, terrestrial planets, where atmospheric temperatures are high, low-mass hydrogen molecules and helium atoms move so swiftly that they can escape from the relatively weak gravity of these tiny planets. Hence, the atmospheres that surround the inner planets are composed primarily of more massive, slower-moving molecules such as CO2, N2, O2, and water vapor (H2O). On the four outer, Jovian planets, low temperatures and relatively strong gravitational attraction with molecules prevent even lightweight hydrogen and helium gases from escaping into space, and so their atmospheres are much more extensive. The combined mass of Jupiter’s atmosphere, for example, is about a million (106) times greater than that of Earth’s atmosphere. This is comparable to the mass of the entire Earth!
Question
ConceptCheck 4-7: Why were astronomers surprised when they first discovered giant Jupiterlike planets orbiting very close to other stars?