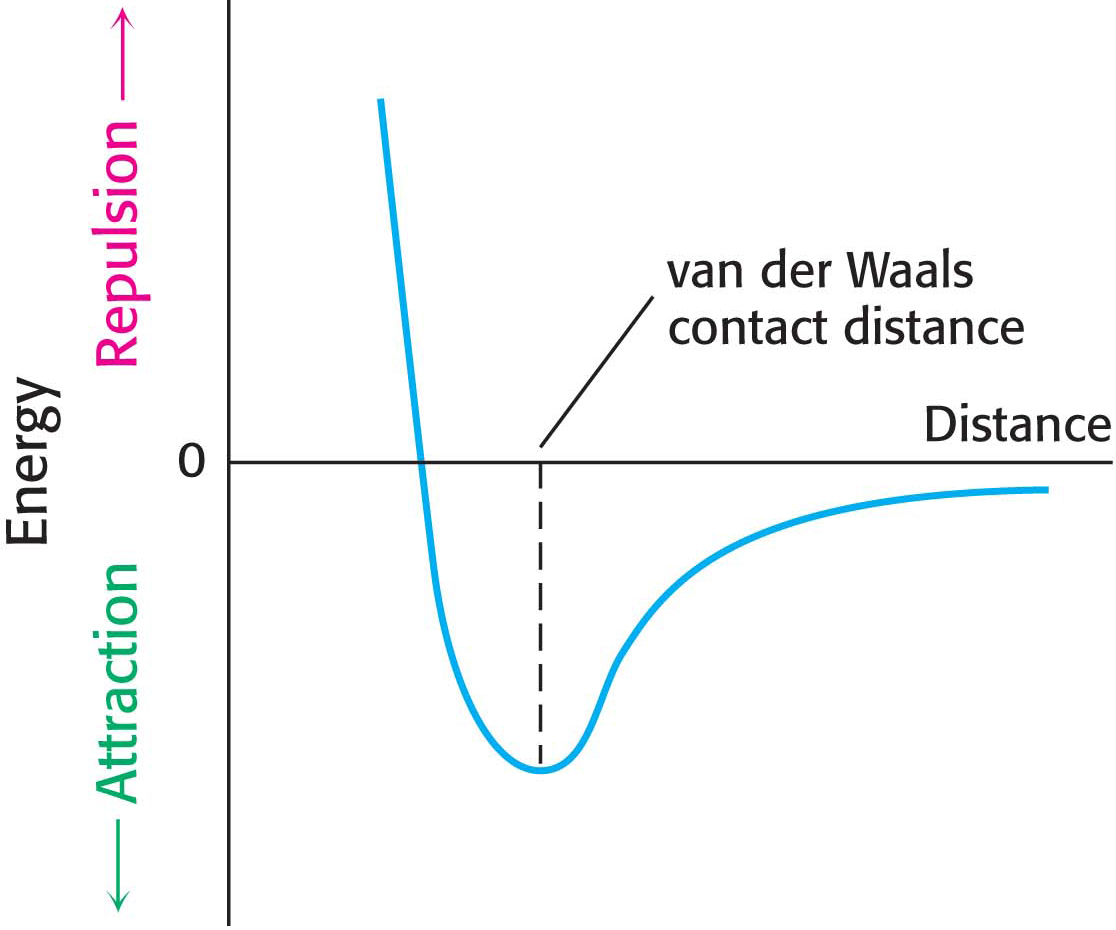
Figure 2.6 The energy of a van der Waals interaction as two atoms approach each other. The energy is most favorable at the van der Waals contact distance. The energy rises rapidly owing to electron–