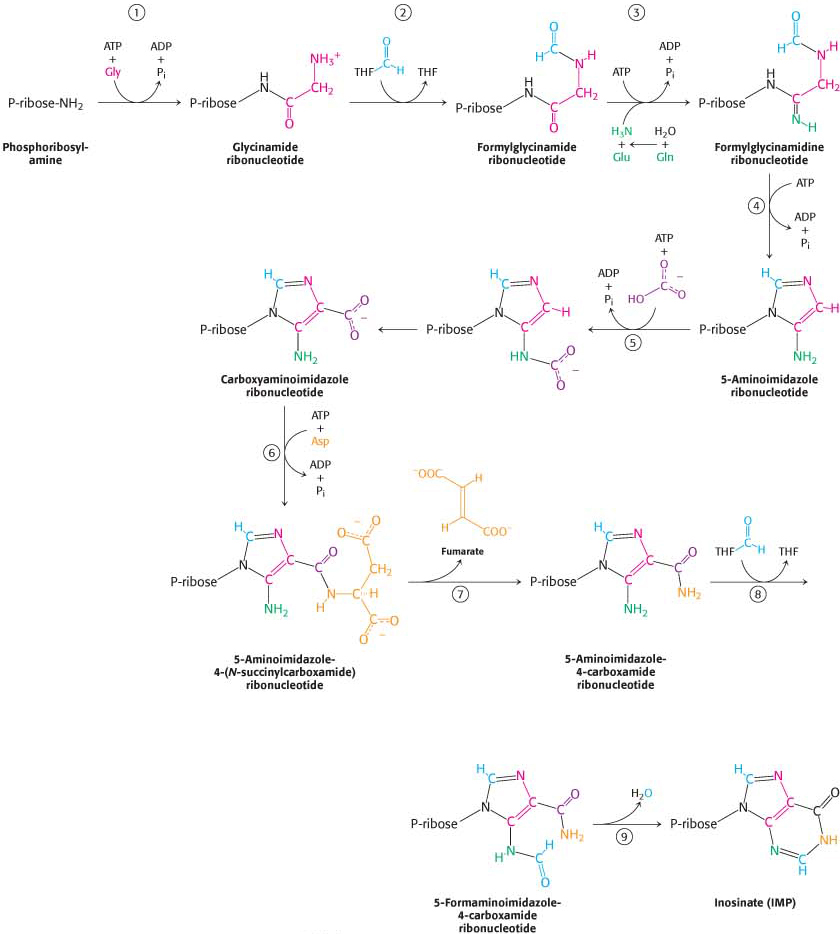
Figure 32.5 De novo purine biosynthesis. (1) Glycine is coupled to the amino group of phosphoribosylamine. (2) N10-Formyltetrahydrofolate (THF) transfers a formyl group to the amino group of the glycine residue. (3) The inner amide group is phosphorylated and then converted into an amidine by the addition of ammonia derived from glutamine. (4) An intramolecular coupling reaction forms a five-