Chapter 13
Complete the interactive matching exercise to see answers.
(1) G-
protein coupled (seven- transmembrane- helix) receptors; (2) receptors that dimerize on ligand binding and recruit tyrosine kinases; (3) receptors that dimerize on ligand binding that are tyrosine kinases (receptor tyrosine kinases) The initial signal—
the binding of the hormone by a receptor— is amplified by enzymes and channels. The receptor must have a site on the extracellular side of the membrane to which the signal molecule can bind and must have an intracellular domain. Binding of the signal to the receptor must induce structural changes on the intracellular domain so that the signal can be transmitted.
The GTPase activity terminates the signal. Without such activity, after a pathway has been activated, it remains activated and is unresponsive to changes in the initial signal.
The presence of the appropriate receptor
Complete the interactive matching exercise to see answers.
The insulin receptor and the EGF receptor employ a common mechanism of signal transmission across the plasma membrane.
Growth-
factor receptors can be activated by dimerization. If an antibody causes the growth- factor receptor to dimerize, the signal- transduction pathway in a cell will be activated. Heterotrimeric G proteins are composed of αβγ subunits. The α subunit contains the GTP-
binding site. On activation by the signal- receptor event, the GDP is exchanged for GTP, and the βγ subunits dissociate from the α subunit bound to GTP, which then activates other pathway components such as adenylate cyclase. Small G proteins, such as Ras, are single- subunit proteins. They are activated by proteins such as Sos in the EGF signal pathway. The activation causes the exchange of GDP for GTP to activate Ras, which in turn, actives specific kinases. The mutated α subunit would always be in the GTP form and, hence, in the active form, which would stimulate its signaling pathway.
Calcium ions diffuse slowly because they bind to many protein surfaces within a cell, impeding their free motion. Cyclic AMP does not bind as frequently, and so it diffuses more rapidly.
Gαs stimulates adenylate cyclase, leading to the generation of cAMP. This signal then leads to glucose mobilization (Chapter 24). If cAMP phosphodiesterase were inhibited, then cAMP levels would remain high even after the termination of the epinephrine signal, and glucose mobilization would continue.
The full network of pathways initiated by insulin includes a large number of proteins and is substantially more elaborate than indicated in Figure 13.18. Furthermore, many additional proteins take part in the termination of insulin signaling. A defect in any of the proteins in the insulin-
signaling pathways or in the subsequent termination of the insulin response could potentially cause problems. Therefore, it is not surprising that many different gene defects can cause type 2 diabetes. The binding of growth hormone causes its monomeric receptor to dimerize. The dimeric receptor can then activate a separate tyrosine kinase to which the receptor binds. The signaling pathway can then continue in similar fashion to the pathways that are activated by the insulin receptor or other mammalian EGF receptors.
C14
Proto-
oncogenes are normally expressed versions of genes that encode proteins that usually regulate cell growth. Oncogenes are proto- oncogenes that are mutated or overexpressed such that the encoded protein always enhances growth. Tumor- suppressor genes encode proteins that inhibit cell growth or induce cell death. Proto-
oncogenes often initiate or are components of pathways leading to cell growth and division in response to some sort of signal. If only one gene is mutated, the cell will be continuously stimulated to grow, even if the other gene continues to function normally. On the other hand, tumor- suppressor genes inhibit the growth signals in some fashion. Thus, even if one gene is nonfunctional, the remaining normal gene is usually sufficient to inhibit unrestricted growth. Other potential drug targets within the EGF signaling cascade include, but are not limited to, the kinase active sites of the EGF receptor, Grb-
2, Sos, Ras or any other downstream components of the signaling pathway. Like the receptors discussed in this chapter, ligand-
gated channels bind signal molecules, which alters their activity so as to propagate a signal. For example, the IP3-activated calcium channel is closed until it binds IP3. Recall that hydrophobic residues are rarely exposed to the aqueous environment of the cells. The exposure of such residues allows calmodulin to bind other proteins and thus propagate the signal.
A G protein was a component of the signal-
transduction pathway. The terminal sulfate must not be an effective substrate for the GTPase activity of the G protein. Two identical receptors must recognize different aspects of the same signal molecule.
The negatively charged glutamate residues mimic the negatively charged phosphoserine or phosphothreonine residues and stabilize the active conformation of the enzyme.
Calcium ion levels are kept low by transport systems that extrude Ca2+ from the cell. Given this low steady-
state level, transient Ca2+ increases in intracellular concentration produced by signaling events can be readily sensed. The truncated receptor will dimerize with the full-
length monomers on EGF binding, but cross- phosphorylation cannot take place, because the truncated receptor possesses neither the substrate for the neighboring kinase domain nor its own kinase domain to phosphorylate the C- terminal tail of the other monomer. Hence, these mutant receptors will block normal EGF signaling. 105
X ≈ 10−7 M; Y ≈ 5 × 10−6 M; Z ≈ 10−3 M
Because much less × is required to fill half of the sites, × displays the highest affinity.
The binding affinity almost perfectly matches the ability to stimulate adenylate cyclase, suggesting that the hormone–
receptor complex leads to the stimulation of adenylate cyclase. Try performing the experiment in the presence of antibodies to Gαs.
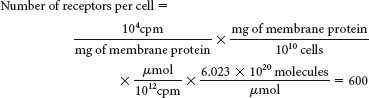
The total binding does not distinguish binding to a specific receptor from binding to different receptors or from nonspecific binding to the membrane.
The rationale is that the receptor will have a high affinity for the ligand. Thus, in the presence of excess nonradioactive ligand, the receptor will bind to nonradioactive ligand. Therefore, any binding of the radioactive ligand must be nonspecific.
The plateau suggests that the number of receptor-
binding sites in the cell membrane is limited.