Chapter 25
Complete the interactive matching exercise to see answers.
Phosphoglucomutase, UDP-
glucose pyrophosphorylase, pyrophosphatase, glycogenin, glycogen synthase, and branching enzyme 
The enzyme pyrophosphatase converts the pyrophosphate into two molecules of inorganic phosphate. This conversion renders the overall reaction irreversible.

The presence of high concentrations of glucose 6-
phosphate indicates that glucose is abundant and that it is not being used by glycolysis. Therefore, this valuable resource is saved by incorporation into glycogen. Glycogenin, a dimer, catalyzes the addition of 10–
20 glucosyl units linked by α-1,4 bonds to each glycogenin subunit. The modified glycogenin then serves as a primer for glycogen synthase, which extends the glycogen chain with the formation of more α-1,4 linkages. C26
Free glucose must be phosphorylated at the expense of a molecule of ATP. Glucose 6-
phosphate derived from glycogen is formed by phosphorolytic cleavage, sparing one molecule of ATP. Thus, the net yield of ATP when glycogen- derived glucose is processed to pyruvate is three molecules of ATP compared with two molecules of ATP from free glucose. Breakdown: Phosphoglucomutase converts glucose 1-
phosphate, liberated from glycogen breakdown, into glucose 6- phosphate, which can be released as free glucose (liver) or processed in glycolysis (muscle and liver). Synthesis: Converts glucose 6- phosphate into glucose 1- phosphate, which reacts with UTP to form UDP- glucose, the substrate for glycogen synthase. 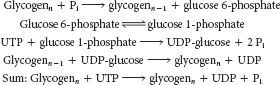
In principle, having glycogen be the only primer for the further synthesis of glycogen should be a successful strategy. However, if the glycogen granules were not evenly divided between daughter cells, glycogen stores for future generations of cells might be compromised. Glycogenin synthesizes the primer for glycogen synthase.
Insulin binds to its receptor and activates the tyrosine kinase activity of the receptor, which in turn triggers a pathway that activates protein kinases. The signal-
transduction pathway results in an increase in the number of glucose transporters in the membrane. Moreover, the kinases phosphorylate and inactivate glycogen synthase kinase. Protein phosphatase 1 then removes the phosphate from glycogen synthase and thereby activates the synthase. The high concentration of glucose 6-
phosphate in von Gierke disease, resulting from the absence of glucose 6- phosphatase or the transporter, shifts the allosteric equilibrium of phosphorylated glycogen synthase toward the active form. Muscle phosphorylase b will be inactive even when the concentration of AMP is high. Hence, glycogen will not be degraded unless phosphorylase is converted into the a form by hormone-
induced or Ca2+-induced phosphorylation. Phosphorylase b cannot be converted into the much more active a form. Hence, the mobilization of liver glycogen will be markedly impaired.
The elevated amount of the kinase will lead to the phosphorylation and activation of glycogen phosphorylase. Because glycogen will be persistently degraded, little glycogen will be present in the liver.
Protein phosphatase 1 will be continually active. Hence, the amount of phosphorylase b will be higher than normal, and glycogen will be less readily degraded.
Protein phosphatase 1 will be much less effective in dephosphorylating glycogen synthase and glycogen phosphorylase. Consequently, the synthase will stay in the less active b form, and the phosphorylase will stay in the more active a form. Both changes will lead to increased degradation of glycogen.
The absence of glycogenin will prevent the initiation of glycogen synthesis. Very little glycogen will be synthesized in its absence.
The α subunit will thus always be active. Cyclic AMP will always be produced. Glycogen will always be degraded, and glycogen synthesis will always be inhibited.
Phosphodiesterase destroys cAMP. Therefore, glycogen degradation will always be active and glycogen synthesis will always be inhibited.
This disease can also be produced by a mutation in the gene that encodes the glucose 6-
phosphate transporter. Recall that glucose 6- phosphate must be transported into the lumen of the endoplasmic reticulum to be hydrolyzed by phosphatase. Mutations in the other essential proteins of this system can likewise lead to von Gierke disease. Glucagon stimulates glycogen breakdown, and the product of debranching enzyme is free glucose, which is released into the blood (≈10% of available glucose in glycogen is contained in α-1,6 branch points).
Galactose is converted into UDP-
galactose to eventually form glucose 6- phosphate. 
The amylase activity was necessary for the removal of all of the glycogen from glycogenin. Recall that glycogenin synthesizes oligosaccharides of about 10–
20 glucose units, and then activity stops. Consequently, if the glucose residues are not removed by extensive amylase treatment, glycogenin will not be detected. The patient has a deficiency of the branching enzyme.
Glycogen was too large to enter the gel, and, because analysis was by western blot with the use of an antibody specific to glycogenin, we would not expect to see background proteins.
α-Amylase degrades glycogen, releasing the protein glycogenin, which can be visualized by the western blot.
Glycogen phosphorylase, glycogen synthase, and protein phosphatase 1. These proteins might be visible if the gels were stained for protein, but a western analysis reveals the presence of glycogenin only.
The smear was due to molecules of glycogenin with increasingly large amounts of glycogen attached to them.
In the absence of glucose in the medium, glycogen is metabolized, resulting in a loss of the high-
molecular- weight material. Glycogen could have been resynthesized and added to the glycogenin when the cells were fed glucose again.
No difference between lanes 3 and 4 suggests that, by 1 hour, the glycogen molecules had attained maximum size in this cell line. Prolonged incubation does not apparently increase the amount of glycogen.
α-Amylase removes essentially all of the glycogen, and so only the glycogenin remains.