Chapter 6
Rate enhancement and substrate specificity
The active site is a three-
dimensional crevice or cleft; it makes up only a small part of the total volume of the enzyme. Active sites have unique microenvironments. A substrate binds to the active site with multiple weak interactions. The specificity of the active site depends on the active site’s precise three- dimensional structure. A cofactor
Coenzymes and metals
Vitamins are converted into coenzymes that are required for most biochemical reactions.
Enzymes facilitate the formation of the transition state.
C6
The intricate three-
dimensional structure of proteins allows the construction of active sites that will recognize only specific substrates. Binding energy is the free energy released when two molecules bind together, such as when an enzyme and a substrate interact.
Binding energy is maximized when an enzyme interacts with the transition state, thereby facilitating the formation of the transition state and enhancing the rate of the reaction.
There would be no catalytic activity. If the enzyme–
substrate complex is more stable than the enzyme– transition- state complex, the transition state would not form and catalysis would not take place. Complete the interactive matching exercise to see answers.
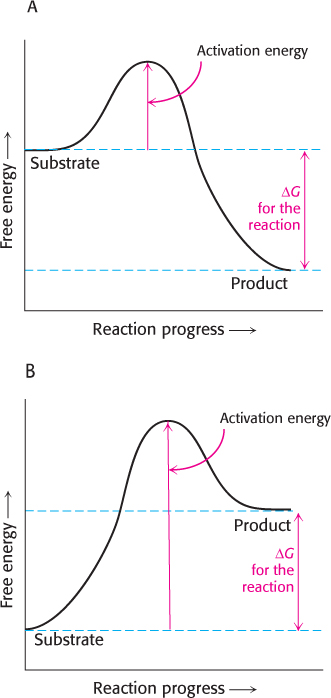
The energy required to reach the transition state (the activation energy) is returned when the transition state proceeds to product.
The product is more stable than the substrate in graph A; so ΔG is negative and the reaction is exergonic. In graph B, the product has more energy than the substrate has; ΔG is positive, meaning that the reaction is endergonic.
Protein hydrolysis has a large activation energy. Protein synthesis requires energy to proceed.
Lysozyme helps protect the fluid that surrounds eyes from bacterial infection.
Transition states are very unstable. Consequently, molecules that resemble transition states are themselves likely to be unstable and, hence, difficult to synthesize.
Complete the interactive matching exercise to see answers.
Keq = 19, ΔG°′ = −7.41 kJ mol−1 (−1.77 kcal mol−1)
This reaction takes place in glycolysis (Chapter 16). At equilibrium, the ratio of GAP to DHAP is 0.0475 at 25°C (298 K) and pH 7. Hence, K′eq = 0.0475. The standard free-
energy change for this reaction is then calculated from equation 5: 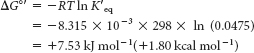
Under these conditions, the reaction is endergonic. DHAP will not spontaneously convert into GAP.
Substituting these values into equation 1 gives
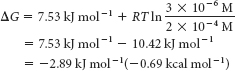
This negative value for ΔG indicates that the isomerization of DHAP to GAP is exergonic and can take place spontaneously when these species are present at the preceding concentrations. Note that ΔG for this reaction is negative, although ΔG°′ is positive.
The mutation slows the reaction by a factor of 100. The activation-
free energy is increased by 11.4 kJ mol−1 (2.73 kcal mol−1). Strong binding of the substrate relative to the transition state slows catalysis. Incubating the enzyme at 37°C leads to the denaturation of enzyme structure and a loss of activity. For this reason, most enzymes must be kept cool if they are not actively catalyzing their reactions.
The coenzyme apparently helps to stabilize the enzyme’s structure, because enzyme from PLP-
deficient cells denatures faster. Cofactors often help to stabilize enzyme structure.
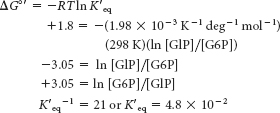
Because [G6P]/[G1P] = 21, there is 1 molecule of G1P for every 21 molecules of G6P. Because we started with 0.1 M, the [G1P] is 1/22(0.1 M) = 0.0045 M and [G6P] must be 21/22(0.1 M), or 0.096 M. The reaction does not proceed to a significant extent as written.Supply G6P at a high rate, and remove G1P at a high rate by other reactions. In other words, make sure that the [G6P]/[G1P] ratio is kept large.
Potential hydrogen-
bond donors at pH 7 are the side chains of the following residues: arginine, asparagine, glutamine, histidine, lysine, serine, threonine, tryptophan, and tyrosine. 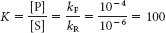 . Using equation 5 in the text, ΔG°′ = −11.42 kJ mol−1 (−2.73 kcal mol−1).
. Using equation 5 in the text, ΔG°′ = −11.42 kJ mol−1 (−2.73 kcal mol−1).kF = 10−2 s−1 and kR = 10−4 s−1. The equilibrium constant and ΔG°′ values are the same for both the uncatalyzed and catalyzed reactions.