Chapter 8
Covalent catalysis; general acid–
base catalysis; metal ion catalysis; catalysis by approximation and orientation The three-
dimensional structure of an enzyme is stabilized by interactions with the substrate, reaction intermediates, and products. This stabilization minimizes thermal denaturation. This piece of information is necessary for determining the correct dosage of succinylcholine to administer.
The duration of the paralysis depends on the ability of the serum cholinesterase to clear the drug. If there were one-
eighth the amount of enzyme activity, paralysis could last eight times as long, which is undesirable for a number of reasons. First, the respirator might break from extended use, which would not be good for the patient on the respirator; second, the doctors might miss their golf game. KM is the concentration needed by the enzyme to reach ½ Vmax. Consequently, for a given concentration of substrate, the reaction catalyzed by the enzyme with the lower KM will have the higher rate. The mutant patient with the higher KM will clear the drug at a much lower rate. Part b describes the consequences.
If a particular amino acid side chain is suspected of participating in a catalytic mechanism, covalent modification of the residue by a group-
specific reagent may alter it sufficiently that the enzyme activity is altered or inhibited. Complete the interactive matching exercise to see answers.
In the absence of inhibitor, Vmax is 47.6 µmol minute−1 and KM is 1.1 × 10−5 M. In the presence of inhibitor, Vmax is the same and the apparent KM is 3.1 × 10−5 M.
Competitive
Vmax is 9.5 µmol minute−1. KM is 1.1 × 10−5 M, the same as without inhibitor.
Noncompetitive
Group-
specific inhibitors; affinity analogs; suicide inhibitors; transition- state analogs The lactam ring of penicillin reacts with a serine residue in glycopeptide transpeptidase, an enzyme that stabilizes the bacterial cell wall. If the lactam were destroyed, penicillin would be ineffective. Indeed, the presence of β-lactamase confers penicillin resistance.
The catalytic triad, composed of the amino acids serine 195, histidine 57, and aspartate 102, resides at the active site of chymotrypsin. The histidine residue serves to position the serine side chain and to polarize its hydroxyl group so that it is poised for deprotonation. In the presence of the substrate, histidine 57 accepts the proton from the serine-
195 hydroxyl group. The withdrawal of the proton from the hydroxyl group generates an alkoxide ion, which is a much more powerful nucleophile than a hydroxyl group is. The aspartate residue helps orient the histidine residue and make it a better proton acceptor through hydrogen bonding and electrostatic effects. C9
The oxyanion hole is a structure at the active site of chymotrypsin that stabilizes the tetrahedral intermediate in the proteolysis reaction and facilitates the formation of the acyl-
enzyme intermediate. Chymotrypsin cleaves peptide bonds in a two-
step reaction, in which the first step, the formation of the acyl- enzyme intermediate, is faster than the second step, hydrolysis. Chymotrypsin recognizes large hydrophobic groups, which are usually buried in the enzyme’s core owing to the hydrophobic effect.
Experimental condition
Vmax
KM
a. Twice as much enzyme is used.
Doubles
No change
b. Half as much enzyme is used.
Half as large
No change
c. A competitive inhibitor is present.
No change
Increases
d. An uncompetitive inhibitor is present.
Decreases
Decreases
e. A pure noncompetitive is present.
Decreases
No change
Because catalysis by chymotrypsin involves a substituted enzyme intermediate, the reaction is a double-
displacement (ping- pong) reaction. When [S+] is much greater than the value of KM, pH will have a negligible effect on the enzyme because S+ will interact with E− as soon as the enzyme becomes available (left-
hand graph). When [S+] is much less than the value of KM, the plot of V0 versus pH becomes essentially a titration curve for the ionizable groups, with enzyme activity being the titration marker. At low pH, the high concentration of H+ will keep the enzyme in the EH form and inactive. As the pH rises, more and more of the enzyme will be in the E− form and active. At high pH (low H+), all of the enzyme is E− (right-
hand graph). The midpoint on this curve will be the pKa of the ionizable group, which is stated to be pH 6.
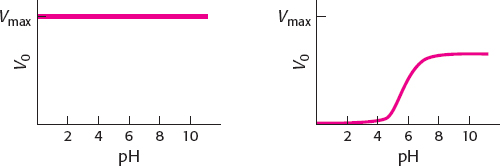
The negative charge on the aspartic acid helps orient histidine 57 so that it acts as a general base catalyst to assist in the formation of the reactive alkoxide ion on serine. Asparagine, lacking a charge, would be less effective at orienting histidine 57. Indeed, chymotrypsin with this mutation has 10,000-
fold lower activity than that of the wild- type enzyme. The formation of the acyl-
enzyme intermediate is slower than the hydrolysis of this amide substrate, and so no burst is observed. For ester substrates, the formation of the acyl- enzyme intermediate is faster. (a) Tosyl-
l-lysine chloromethyl ketone (TLCK). (b) Determine whether substrates protect trypsin from inactivation. Ascertain whether the d isomer of TLCK is an effective inhibitor.