10.1 Monosaccharides Are the Simplest Carbohydrates
✓ 1 Differentiate between monosaccharides and polysaccharides in regard to structure and function.
We begin our consideration of carbohydrates with monosaccharides, the simplest carbohydrates. These simple sugars serve not only as fuel molecules but also as fundamental constituents of living systems. For instance, DNA has a backbone consisting of alternating phosphoryl groups and deoxyribose, a cyclic five-
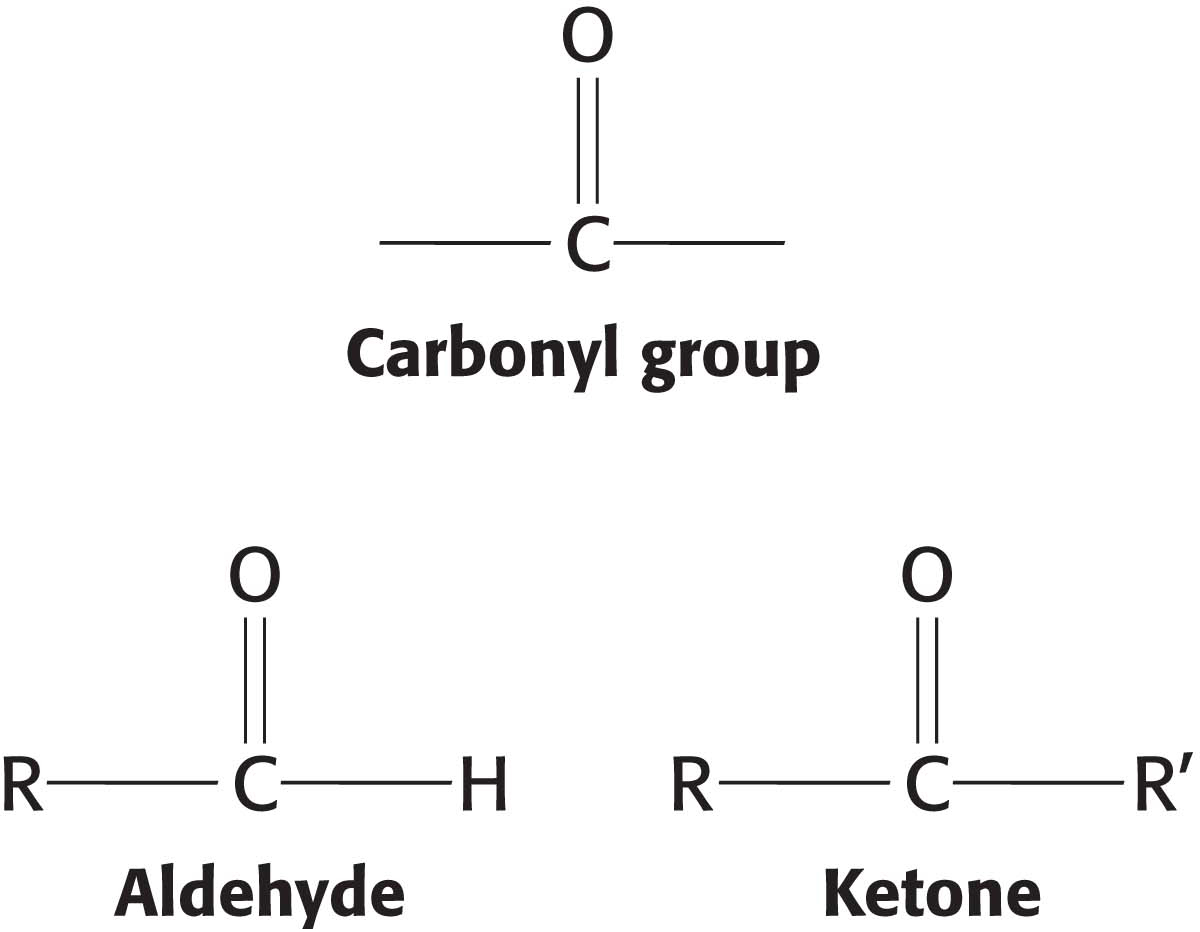
Monosaccharides are aldehydes or ketones that have two or more hydroxyl groups. The smallest monosaccharides, composed of three carbons, are dihydroxyacetone and d- and l-glyceraldehyde.
DID YOU KNOW?
Monosaccharides and other sugars are often represented by Fischer projections. recall from Chapter 3 that, in a Fischer projection of a molecule, atoms joined to an asymmetric tetrahedral carbon atom by horizontal bonds are in front of the plane of the page and those joined by vertical bonds are behind the plane.
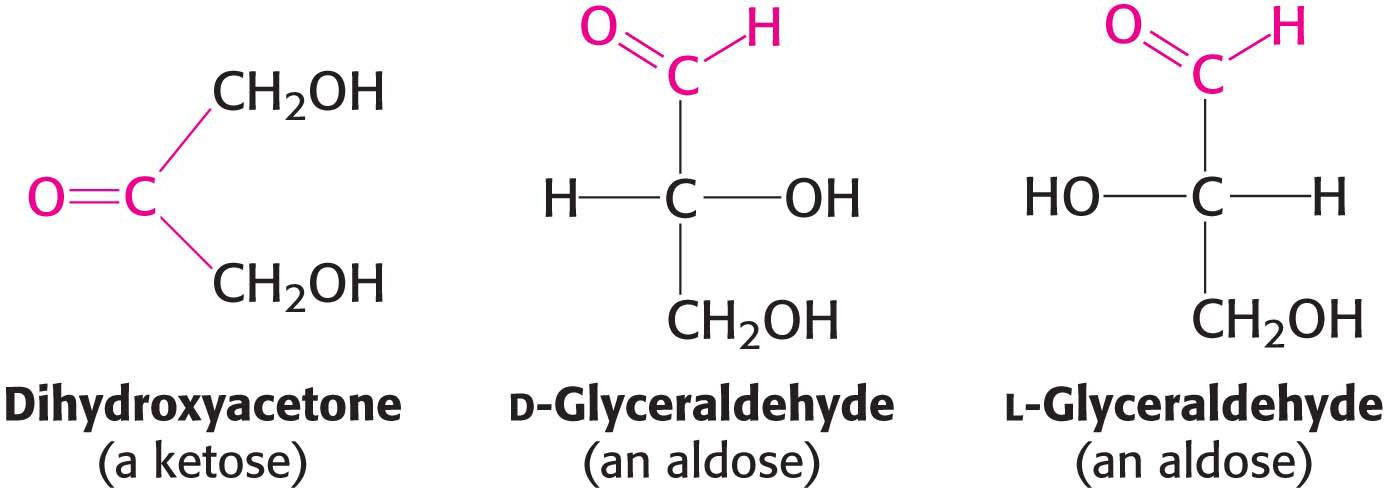
Dihydroxyacetone is called a ketose because it contains a keto group (shown in red), whereas glyceraldehyde is called an aldose because it contains an aldehyde group (also shown in red). They are referred to as trioses (tri-
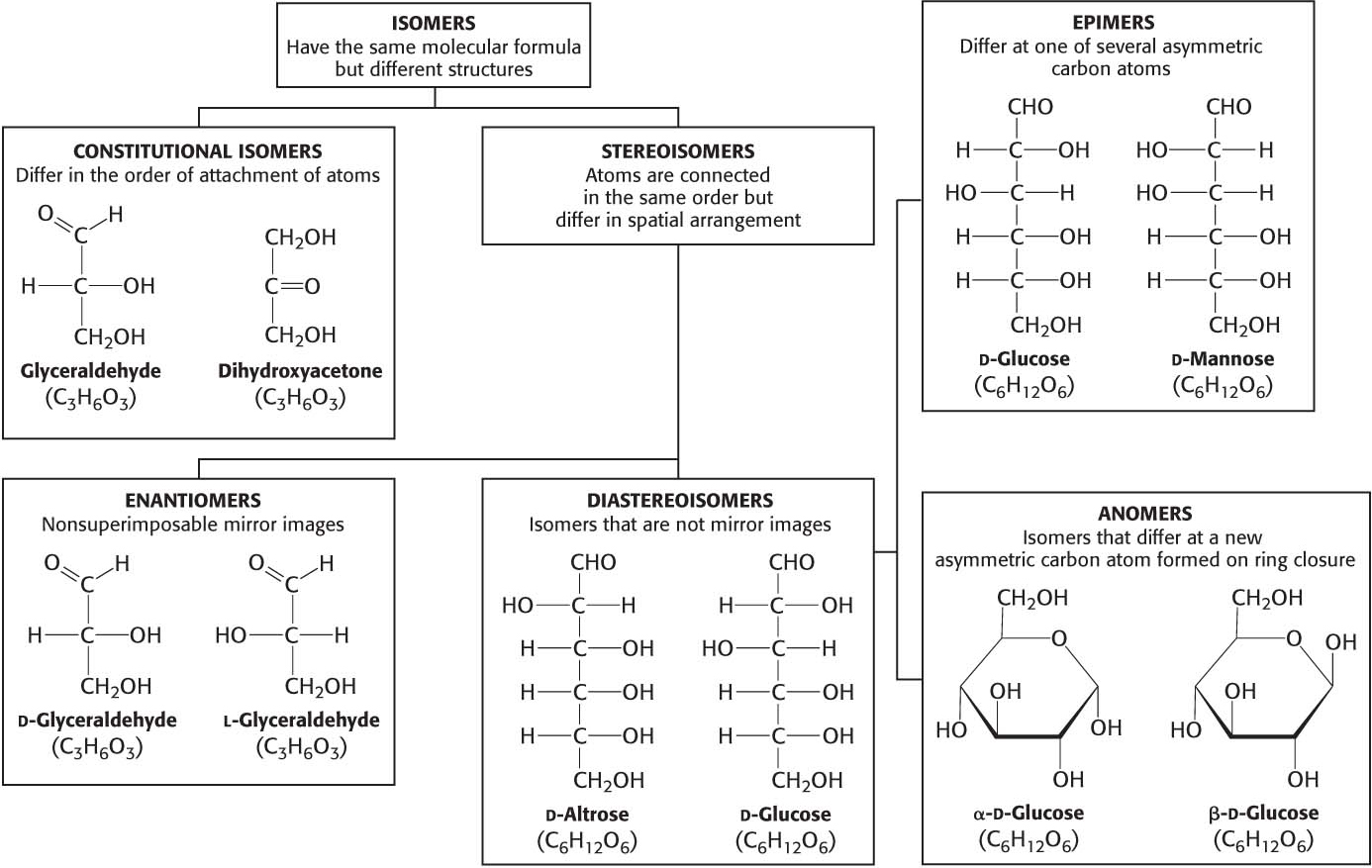
Carbohydrates can exist in a dazzling variety of isomeric forms (Figure 10.1). Dihydroxyacetone and glyceraldehyde are constitutional isomers because they have identical molecular formulas but differ in how the atoms are ordered. Stereoisomers are isomers that differ in spatial arrangement. Glyceraldehyde has a single asymmetric carbon atom, and, thus, there are two stereoisomers of this sugar: d-glyceraldehyde and l-glyceraldehyde. These molecules are a type of stereoisomer called enantiomers, which are mirror images of each other.
169
Monosaccharides made up of more than three carbon atoms have multiple asymmetric carbon atoms, and so they exist not only as enantiomers but also as diastereoisomers, isomers that are not mirror images of each other. According to convention, the d and l isomers are determined by the configuration of the asymmetric carbon atom farthest from the aldehyde or keto group.
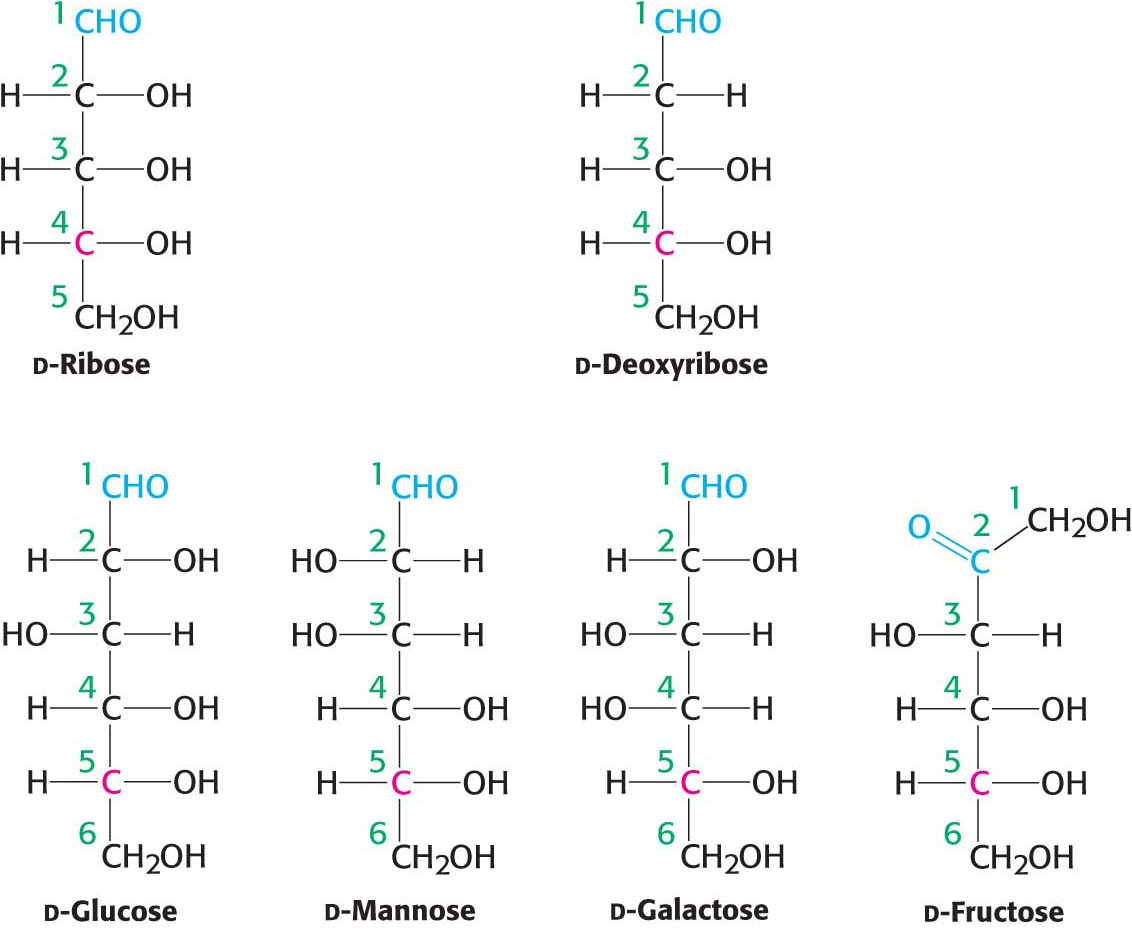
Figure 10.2 shows the common sugars that we will see most frequently in our study of biochemistry. d-Ribose, the carbohydrate component of RNA, is a five-
Many Common Sugars Exist in Cyclic Forms
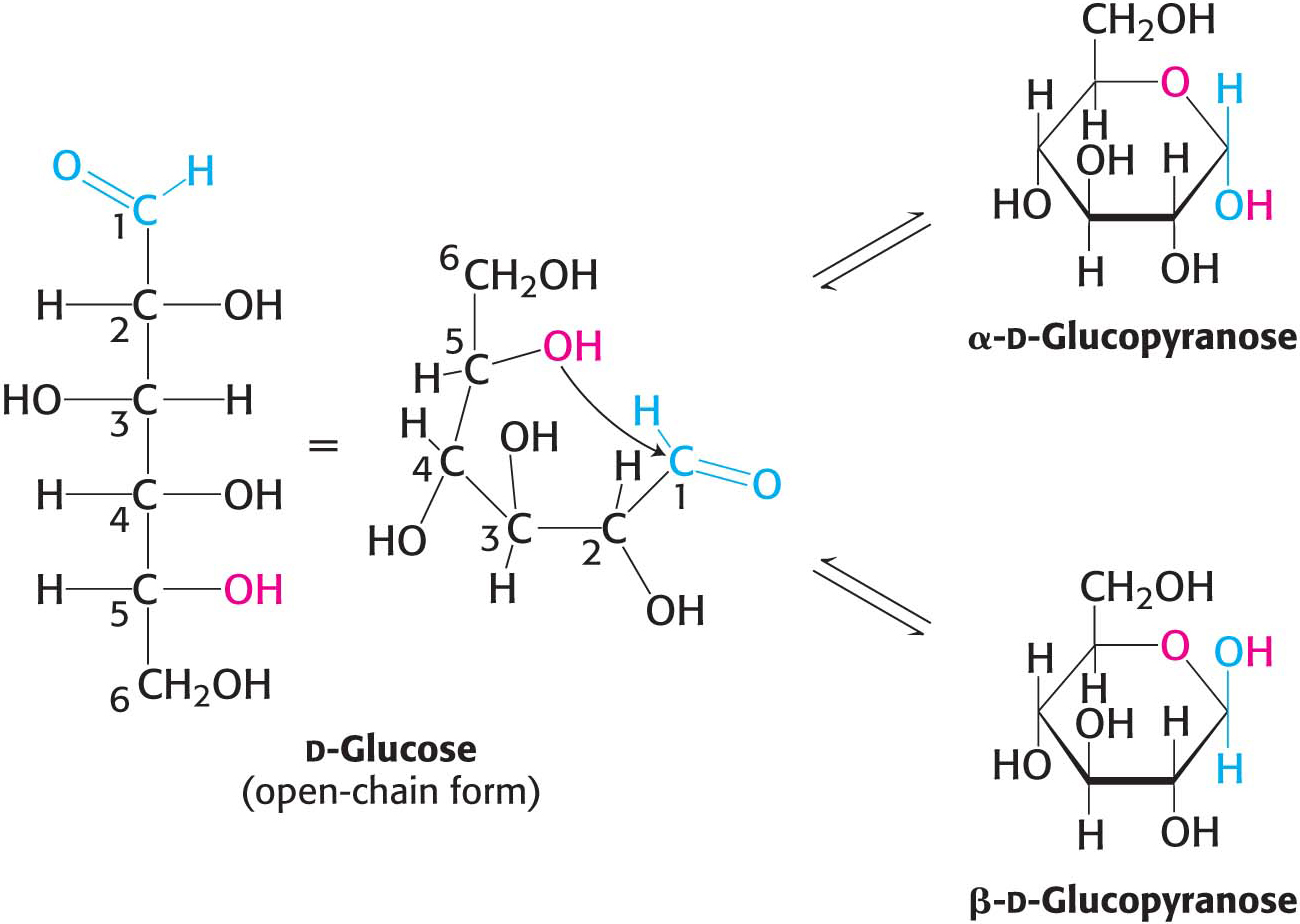
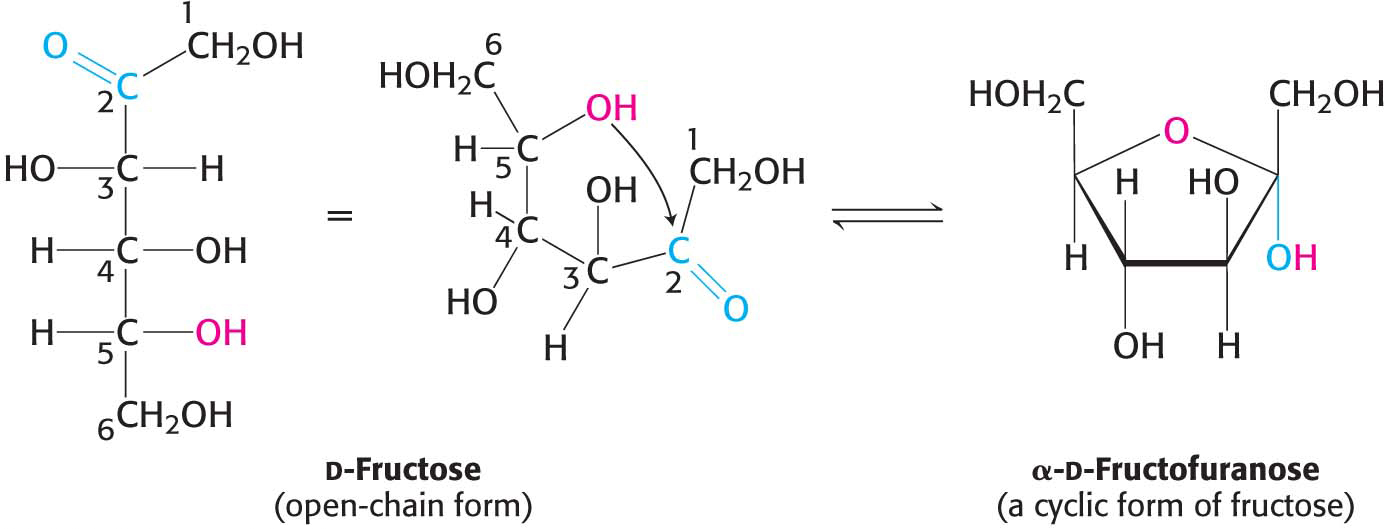
The predominant forms of ribose, glucose, fructose, and many other sugars in solution are not open chains. Rather, the open-
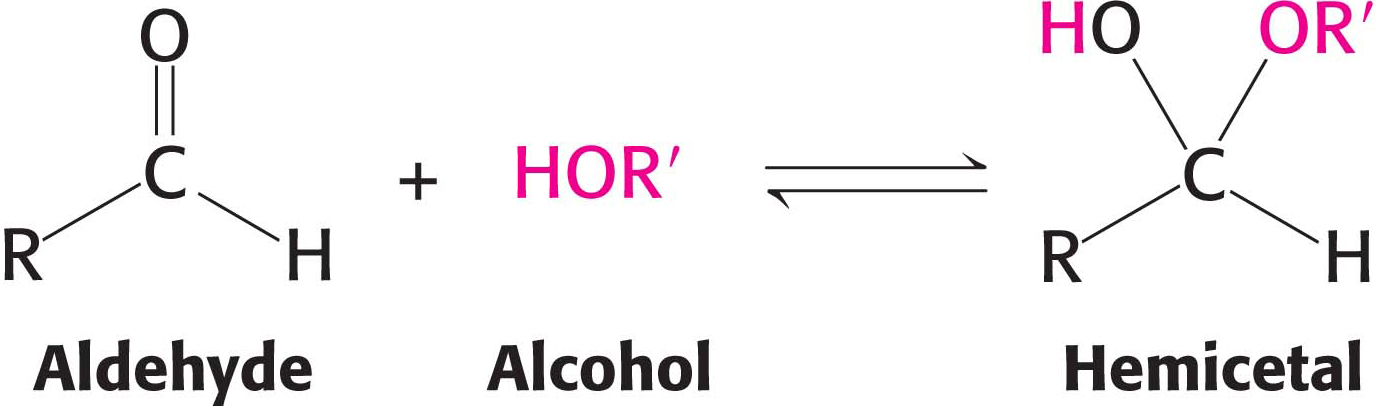
For an aldohexose such as glucose, the same molecule provides both the aldehyde and the alcohol: the C-
170
Similarly, a ketone can react with an alcohol to form a hemiketal.
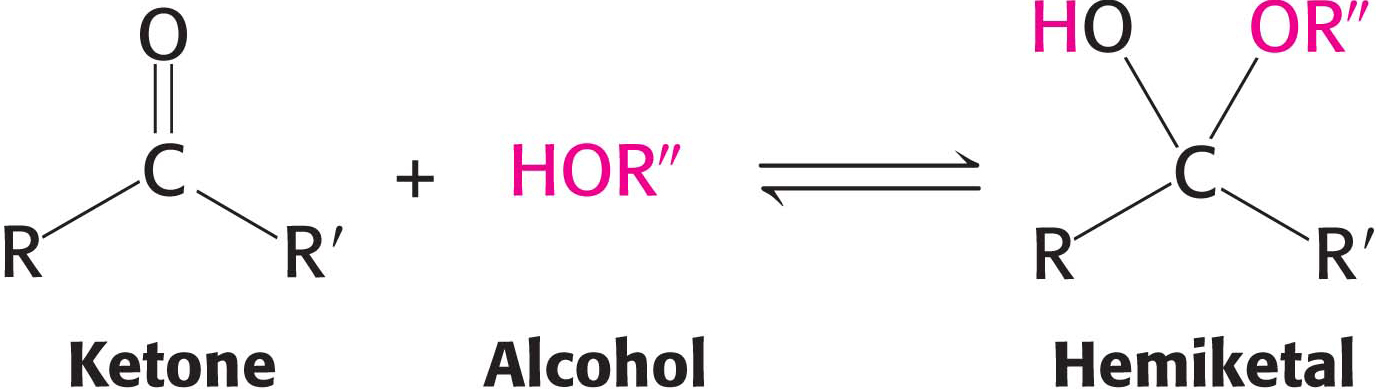
The C-
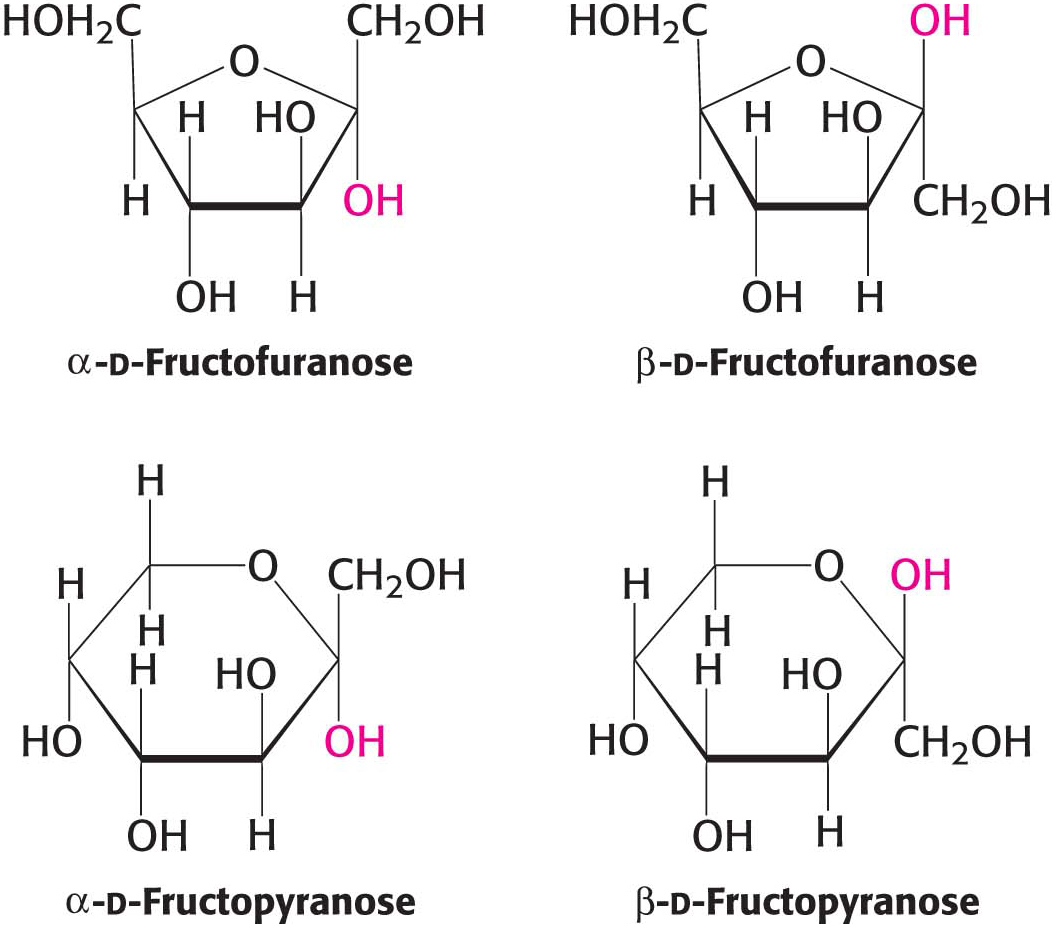
The depictions of glucopyranose (glucose) and fructofuranose (fructose) shown in Figures 10.3 and 10.4 are Haworth projections. In such projections, the carbon atoms in the ring are not written out. The approximate plane of the ring is perpendicular to the plane of the paper, with the heavy line on the ring projecting toward the reader.
We have seen that carbohydrates may contain many asymmetric carbon atoms. An additional asymmetric center is created when a cyclic hemiacetal is formed, generating yet another diastereoisomeric form of sugars called anomers. In glucose, C-
The furanose-
171
β-d-Fructopyranose, found in honey, is one of the sweetest chemicals known. The β-d-fructofuranose form is not nearly as sweet. Heating converts β-fructopyranose into β-fructofuranose, reducing the sweetness of the solution. For this reason, corn syrup with a high concentration of fructose in the β-d-fructopyranose form is used as a sweetener in cold, but not hot, drinks.
Pyranose and Furanose Rings Can Assume Different Conformations
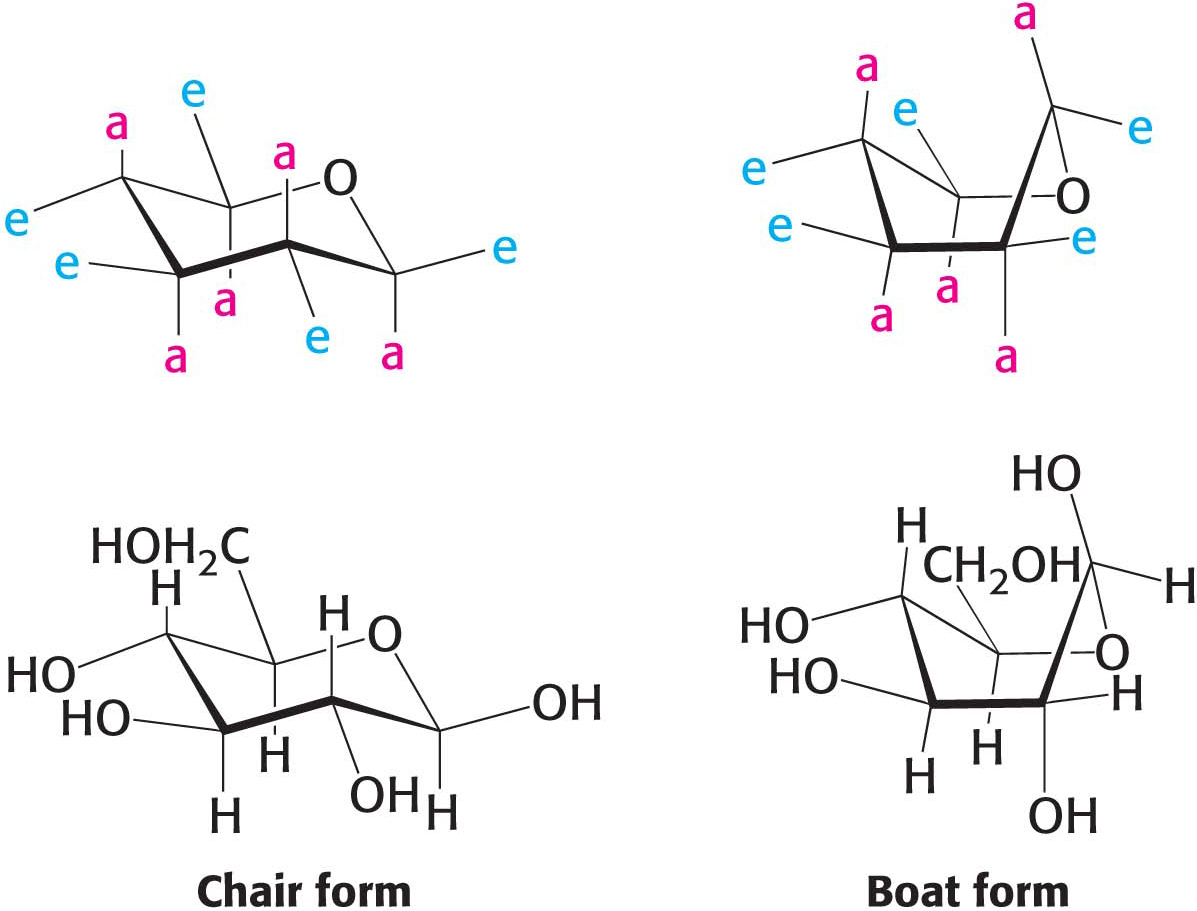
Hayworth projections provide simple means of depicting carbohydrates, but they are misleading in suggesting that the molecules are planar. For instance, stereochemical rendering of the six-
Although both the α and β chair form of d-glucopyranose exist in solution, the chair form of β-d-glucopyranose predominates because all axial positions are occupied by hydrogen atoms. The bulkier −OH and −CH2OH groups emerge at the less hindered periphery. The boat form of glucose is disfavored because it is quite sterically hindered.
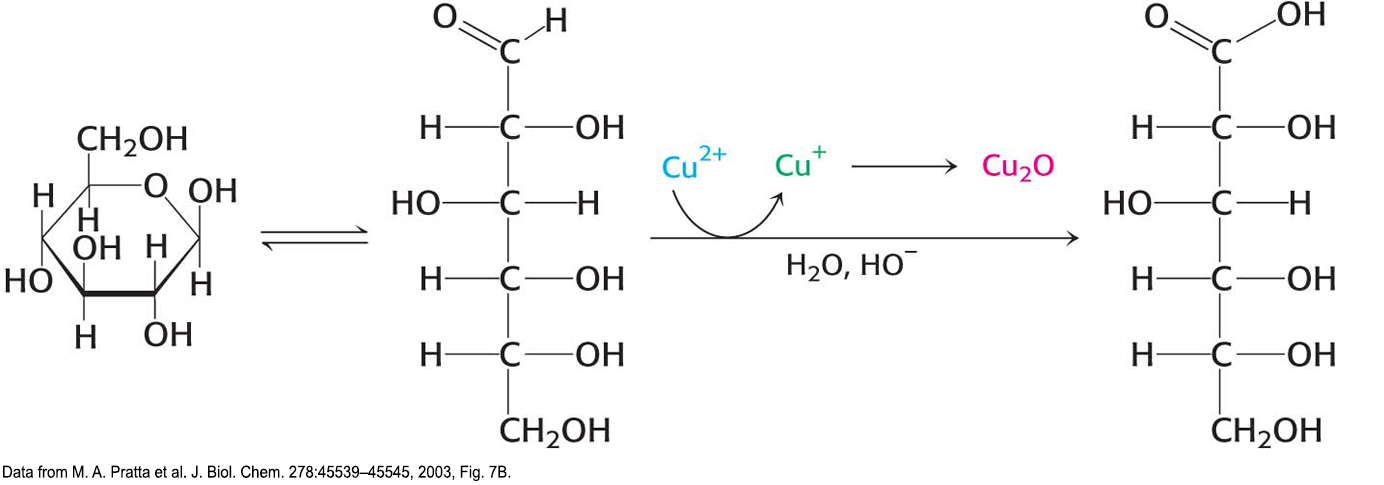
!clinic! CLINICAL INSIGHT: Glucose Is a Reducing Sugar
Because the α and β isomers of glucose are in an equilibrium that passes through the open-
172
Solutions of cupric ion (known as Fehling’s solution) provide a simple test for the presence of sugars such as glucose. Sugars that react with solutions of cupric ion are called reducing sugars; those that do not are called nonreducing sugars. Reducing sugars can often nonspecifically react with amino groups on proteins not participating in a peptide bond. For instance, as a reducing sugar, glucose can react with hemoglobin to form glycosylated hemoglobin (hemoglobin A1c). Changes in the amount of glycosylated hemoglobin can be used to monitor the effectiveness of treatments for diabetes mellitus, a condition characterized by high levels of blood glucose (Chapter 25). Because the glycosylated hemoglobin remains in circulation, the amount of the modified hemoglobin corresponds to the long-
Monosaccharides Are Joined to Alcohols and Amines through Glycosidic Bonds
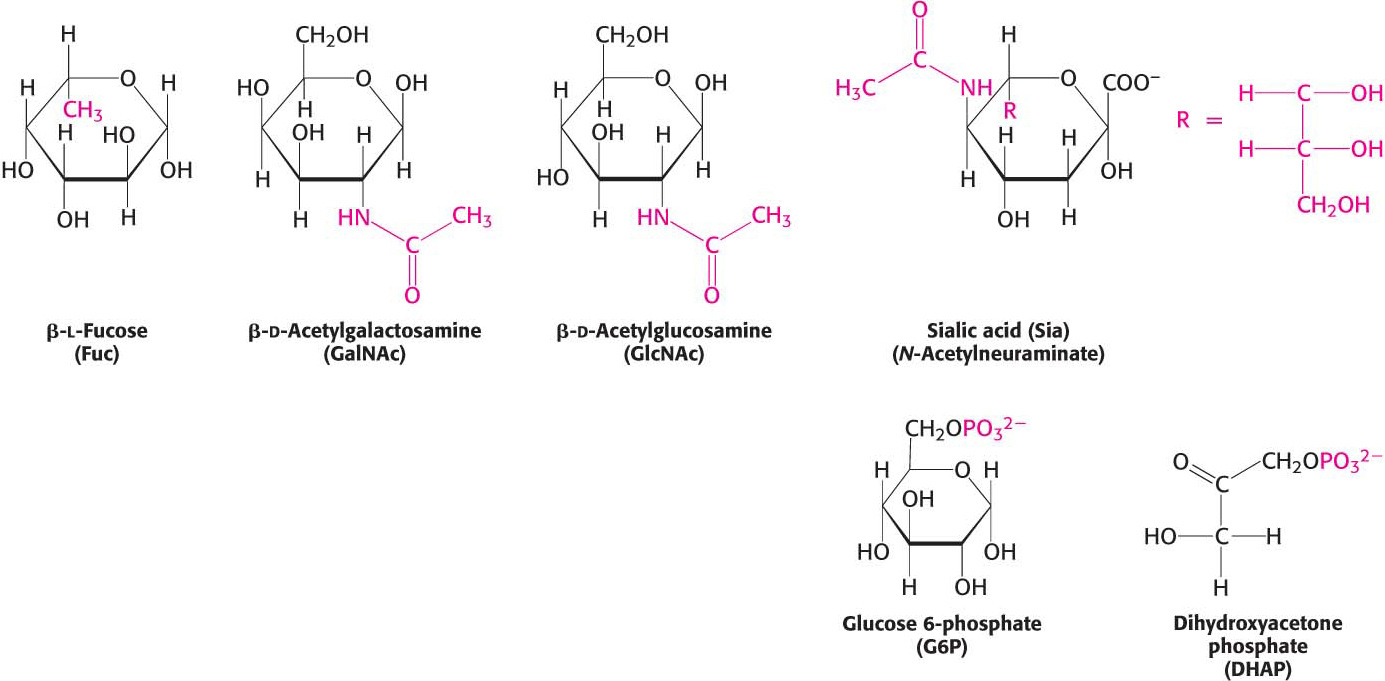
The biochemical properties of monosaccharides can be modified by reaction with other molecules. These modifications increase the biochemical versatility of carbohydrates, enabling them to serve as signal molecules or facilitating their metabolism. Three common reactants are alcohols, amines, and phosphates. A bond formed between the anomeric carbon atom of glucose and the oxygen atom of an alcohol is called a glycosidic bond—specifically, an O-glycosidic bond—and the resulting product is called a glycoside. O-Glycosidic bonds are prominent when carbohydrates are linked together to form long polymers and when they are attached to proteins. In addition, the anomeric carbon atom of a sugar can be linked to the nitrogen atom of an amine to form an N-glycosidic bond. Carbohydrates can also form an ester linkage to phosphates, one of the most prominent modifications in carbohydrate metabolism. For instance, the phosphorylation of glucose is essential when glucose metabolizes to yield ATP (Chapter 16). Examples of modified carbohydrates are shown in Figure 10.7.
173
!bio! BIOLOGICAL INSIGHT: Glucosinolates Protect Plants and Add Flavor to our Diets
Plants, especially those of the order Brassicales, produce a special class of glycosides called glucosinolates as a defense against herbivory. When glucosinolate is hydrolyzed, isothiocyanate is released, generating a sharp taste that discourages further eating by an insect. The glucosinolate is stored separately from the activating enzyme, myrosinase; but on tissue damage, the enzyme and substrate combine for the hydrolysis reaction.
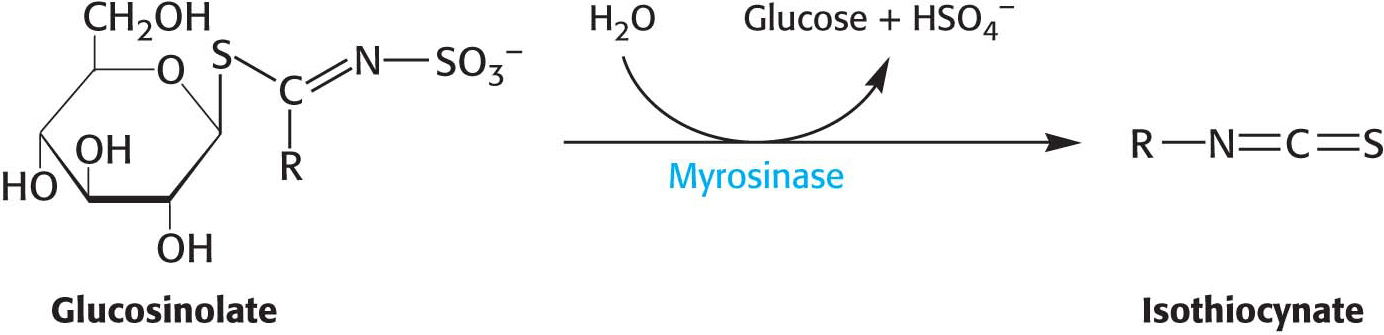
Many glucosinolates vary with the nature of the R component. The combination of the glucosinolate and myrosinase is sometimes called the “mustard-