11.2 Triacylglycerols Are the Storage Form of Fatty Acids
✓ 4 Identify the major lipids, and describe their biochemical functions.

Despite the fact that fatty acids are our principal energy source, the concentration of free fatty acids in cells or the blood is low because free fatty acids are strong acids. High concentrations of free fatty acids would disrupt the pH balance of the cells. Fatty acids required for energy generation are stored as triacylglycerols, which are formed by the attachment of three fatty acid chains to a glycerol molecule. The fatty acids are attached to the glycerol through ester linkages, in a process known as esterification. Common soaps are the sodium or potassium salts of fatty acids generated by treating triacylglycerols with strong bases (Figure 11.2).
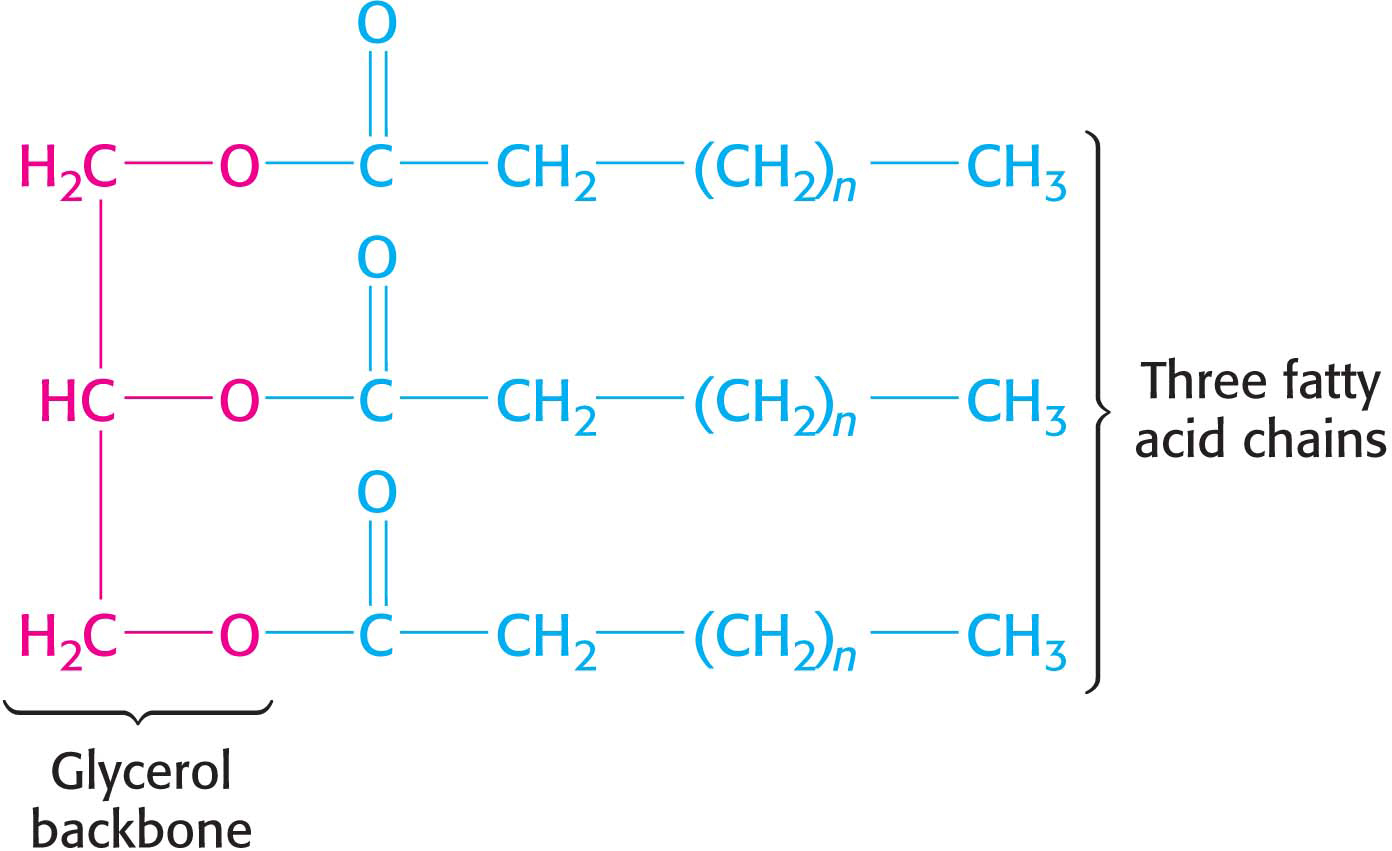
When energy is required during a fast (for instance, while sleeping), the fatty acids are cleaved from the triacylglycerol and carried to the cells. The ingestion of food replenishes the triacylglycerol stores.
As stated in Chapter 10, fatty acids are richer in energy than carbohydrates. Additionally, compared with carbohydrates, fatty acids store energy more efficiently in the form of triacylglycerols, which are hydrophobic and so are stored in a nearly anhydrous form. Polar carbohydrates, in contrast, bind to water molecules. Glycogen, for instance, binds to water. In fact, 1 g of dry glycogen binds about 2 g of water. Consequently, a gram of nearly anhydrous fat stores more than six times as much energy as a gram of hydrated glycogen. For this reason, triacylglycerols rather than glycogen were selected in evolution as the major energy reservoir. Consider a typical 70-
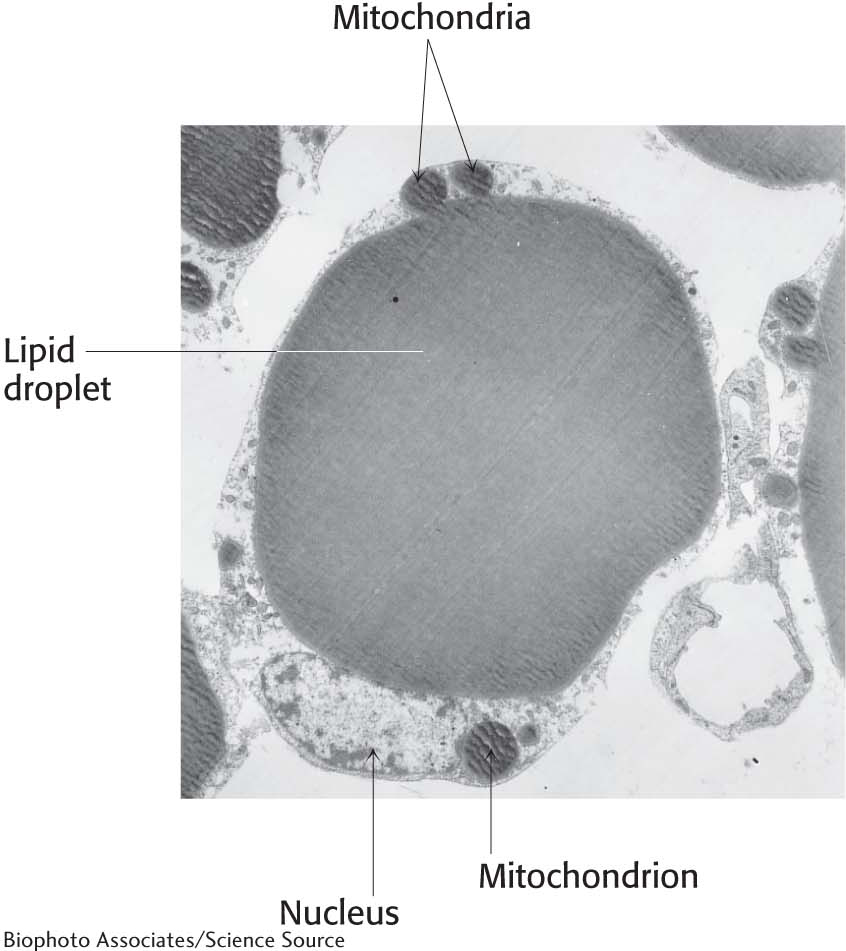
In mammals, the major site of accumulation of triacylglycerols is adipose tissue, which is distributed under the skin and elsewhere throughout the body. In adipose cells (fat cells), droplets of triacylglycerol coalesce to form a large globule in the cytoplasm, which may occupy most of the cell volume (Figure 11.3). Adipose cells are specialized for the synthesis and storage of triacylglycerols and for their mobilization into fuel molecules that are transported to other tissues by the blood. Adipose tissue also serves as a thermal insulator to help maintain body temperature. Polar bears provide an example of the insulating properties of adipose tissue. During fierce arctic storms, polar bears curl up into tight balls and are so well insulated that they are difficult to detect even with heat-
194
The utility of triacylglycerols as an energy source is dramatically illustrated by the abilities of migratory birds, which can fly great distances without eating. Examples are the American golden plover and the ruby-
