14.2 Proteases Digest Proteins into Amino Acids and Peptides
✓ 2 Explain how the release of pancreatic enzymes is coordinated with digestion in the stomach.
Digestion begins in the mouth with the process of chewing, where teeth, tongue, and saliva are employed to homogenize a bite of pizza, converting it into an aqueous slurry that is more readily attacked by digestive enzymes than a piece of poorly chewed food would be.
Saliva, secreted by the salivary glands, is an aqueous solution of Na+, K+, Cl−, and HCO3− that contains mucoproteins. These components facilitate the homogenization of the food and lubricate the resulting slurry for swallowing. Saliva also contains α-amylase, which cleaves α-1,4 glycosidic bonds. Because of the short duration of time that food is in the mouth, polysaccharide breakdown in the mouth is minimal.
!clinic! CLINICAL INSIGHT: Protein Digestion Begins in the Stomach
Human beings ingest about 70 to 100 g of protein daily. In regard to our pizza, the meat and cheese provide most of the protein, which must be degraded so that the individual amino acids will be available for use in metabolic pathways. In addition to proteins in foods, 50 to 100 g of protein per day is sloughed off the cells of the intestine in the wear and tear of digestion; this protein is degraded, and the amino acids are salvaged.
Subsequent to homogenization, the food passes into the stomach, where two principal activities take place. First, the proteins are denatured by the acidic environment of the stomach, where the pH is maintained at values ranging from 1 to 2. This denaturation, caused by the breakage of ionic and hydrogen bonds due to the low pH, renders protein a better substrate for degradation. Second, the process of protein degradation begins in the stomach with the action of the proteolytic enzyme pepsin, as discussed above. The action of pepsin yields protein fragments that will be further degraded by the proteases of the intestine. Pepsin is a remarkable enzyme, displaying optimal activity in the pH range of 1 to 2, conditions so acidic that other proteins are denatured.
249
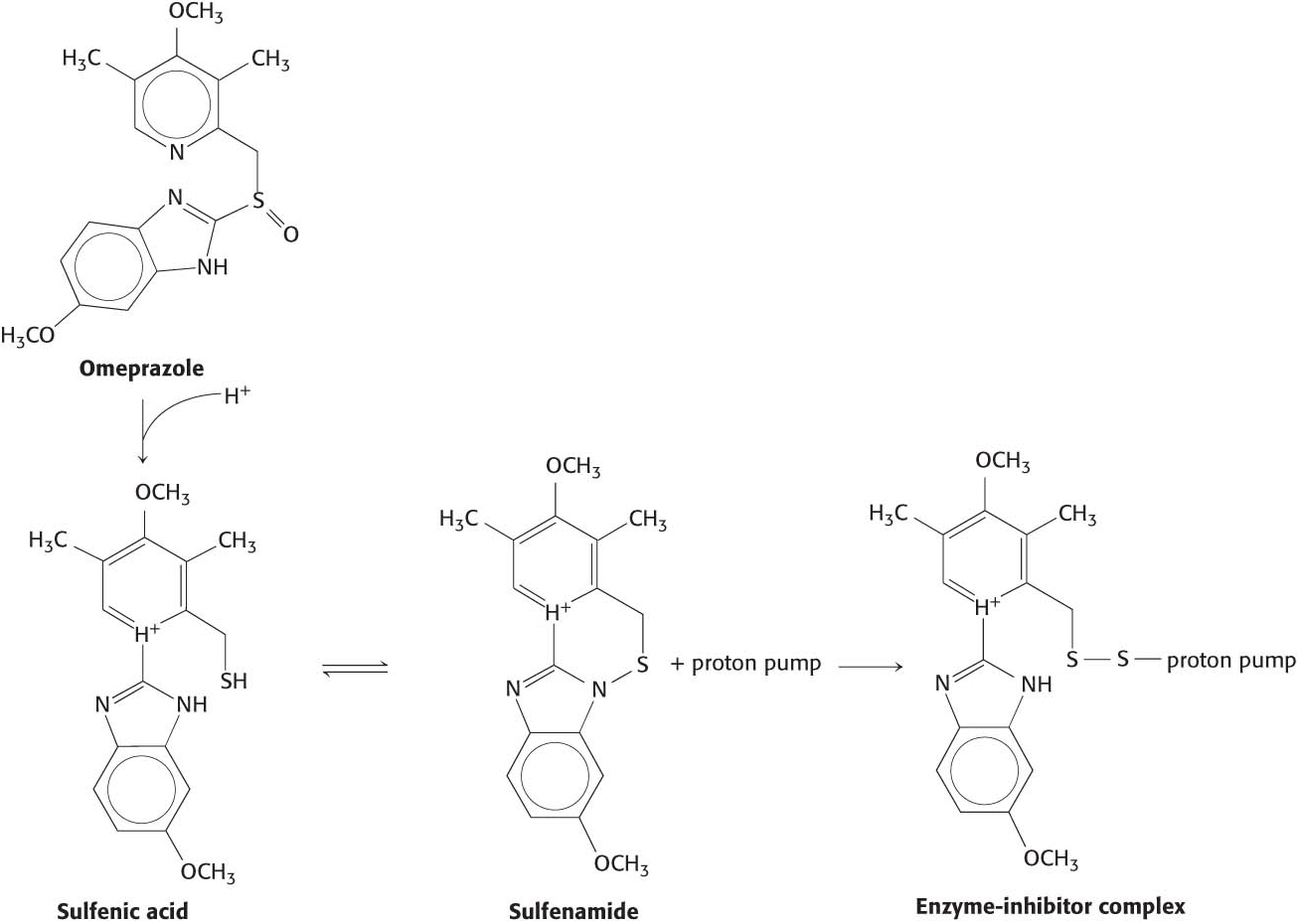
How is the acid environment of the stomach generated? Specialized cells lining the stomach contain the membrane protein K+/H+ ATPase (gastric proton pump) that pumps protons into the stomach in exchange for K+ at the expense of ATP hydrolysis. This proton pump is similar to the Na+–K+ ATPase discussed earlier. In some individuals, the gastric proton pump can be too active, resulting in gastroesophageal reflux disease (GERD, p. 26), a condition where the stomach acid leaks back to the esophagus. In addition to being painful, GERD may eventually result in esophageal cancer if left untreated. A common treatment for GERD is to irreversibly inhibit the proton pump. One such inhibitor, omeprazole, is converted into sulfenic acid by the stomach acid, which rearranges to yield sulfonamide. Sulfonamide irreversibility modifies a cysteine residue on the pump (Figure 14.2). Omeprazole and other proton pump inhibitors are among the most commonly prescribed drugs.
Protein Digestion Continues in the Intestine
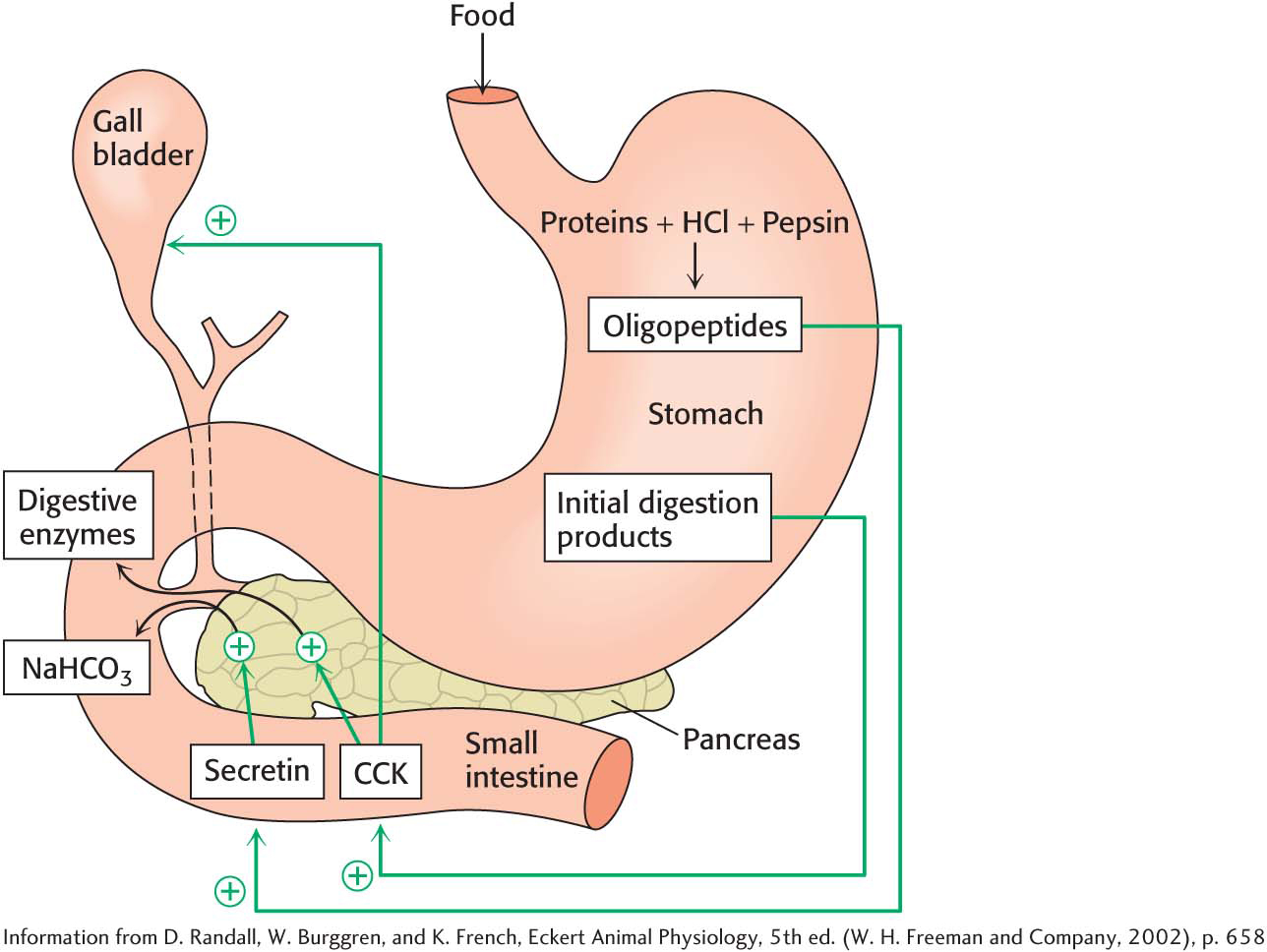
The partly digested proteins as well as carbohydrates and lipids move from the acidic environment of the stomach to the beginning of the small intestine. The low pH of the food stimulates the cells of the small intestine to release the hormone secretin (Figure 14.3). Secretin, in turn, promotes the release of sodium bicarbonate (NaHCO3) from pancreatic cells, which neutralizes the pH of the food as it exits the stomach. The polypeptide products of pepsin digestion also stimulate the release of the hormone cholecystokinin (CCK) by intestinal cells. The pancreas responds to CCK (Figure 14.3) by releasing a host of digestive enzymes into the intestine, where the digestion of proteins continues and the digestion of lipids and carbohydrates begins.
250
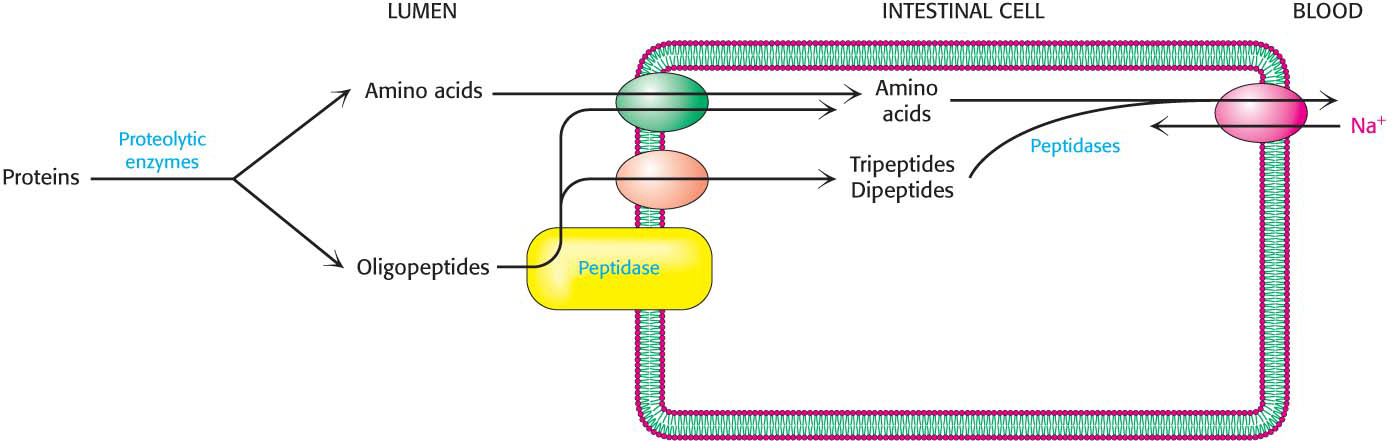
The pancreatic proteases hydrolyze the proteins into small fragments called oligopeptides, but digestion is completed by enzymes called peptidases that are attached to the external surfaces of the intestinal cells. These enzymes cleave the oligopeptides into amino acids and di-
251
!clinic! CLINICAL INSIGHT: Celiac Disease Results from the Inability to Properly Digest Certain Proteins
Celiac disease, also known as gluten enteropathy, is an intestinal inflammatory disorder that is triggered in susceptible individuals by protein in wheat, rye, and barley. Gluten is used as a general term referring to the proteins gliadin and glutenin in wheat and hordein and secalin in barley and rye, respectively. All glutens are rich in the amino acids proline and glutamine and are resistant to complete digestion by pepsin and the pancreatic proteases. Because of their composition, these proteins are sometimes called prolamins. Susceptible individuals are genetically disposed to mount an inflammatory response to gluten-
Glutens are storage proteins in plants, providing amino acids as well as carbon, nitrogen, and sulfur for growth and development. With regard to our pizza, the gluten in the dough gives it elasticity, helping it rise during heating and maintaining its final shape.