14.4 The Digestion of Lipids Is Complicated by Their Hydrophobicity
As we eat our pizza, we may notice that the box has greasy stains. These stains are caused by the lipids in our meal. The main sources of lipids in the pizza are the meat and the cheese, which, as already noted, are sources of protein as well. Most lipids are ingested in the form of triacylglycerols and must be degraded to fatty acids for absorption across the intestinal epithelium. Lipid digestion presents a problem because, unlike carbohydrates and proteins, lipids are not soluble in water. Recall that lipids are highly reduced molecules. This high degree of reduction accounts both for their high energy content and for their poor solubility in water (Chapter 11). How can the lipids be degraded to fatty acids if the lipids are not soluble in the same medium as the degradative enzymes are? Moreover, the lipid-
253
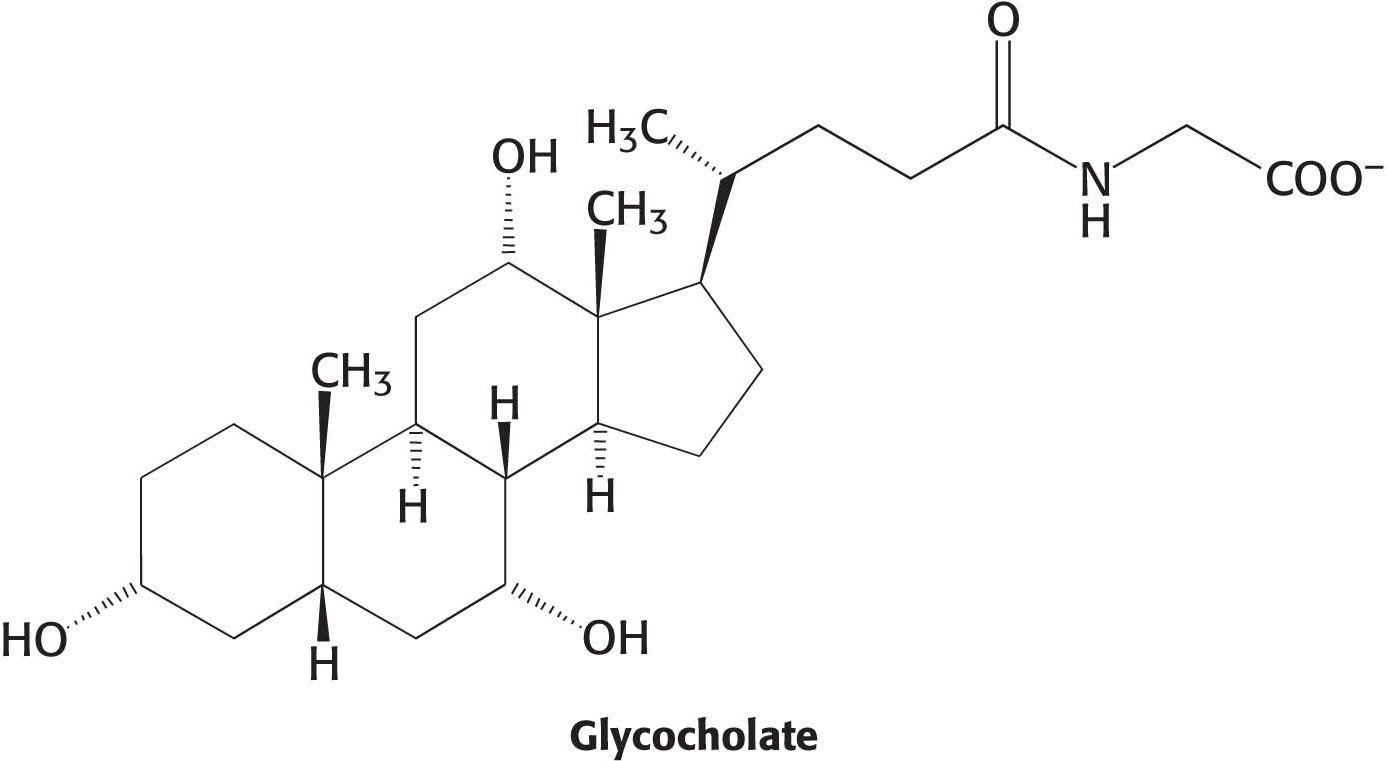
Lipids are prepared for digestion in the stomach. The grinding and mixing that takes place in the stomach converts lipids into an emulsion, a mixture of lipid droplets and water. Common emulsions include mayonnaise and shaken oil-
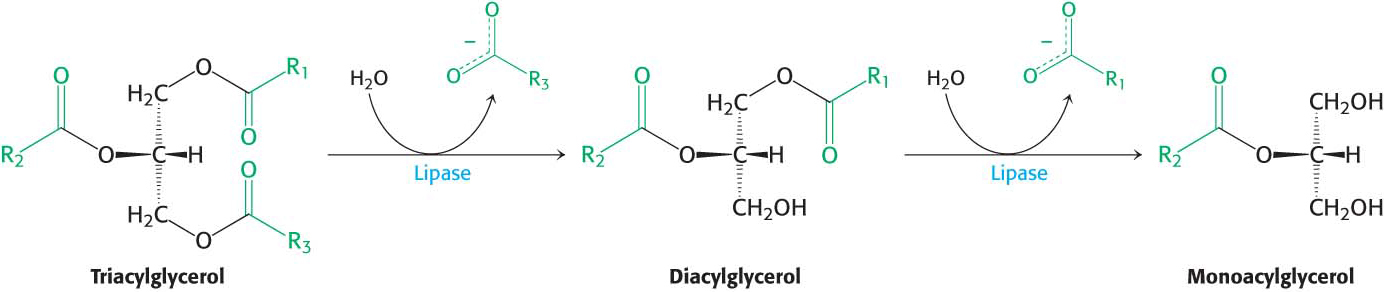
The fatty acids and monoacylglycerol are transported into the intestinal cells by membrane proteins such as the fatty-
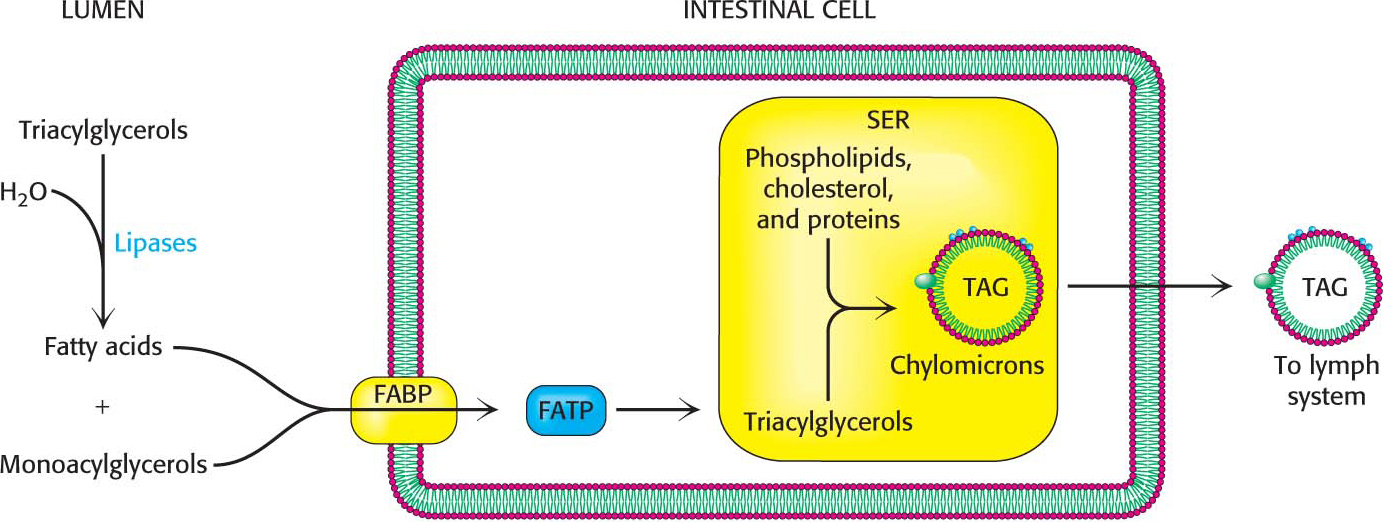
254
!quickquiz! QUICK QUIZ
Explain why a person who has a trypsinogen deficiency will suffer from more digestion difficulties than will a person lacking most other zymogens.
After a meal rich in lipids, the blood appears milky because of the high content of chylomicrons. These particles bind to membrane-

!bio! BIOLOGICAL INSIGHT: Snake Venoms Digest from the Inside Out
Most animals ingest food and, in response to this ingestion, produce enzymes that digest the food. Many venomous snakes, on the other hand, do the opposite. They inject digestive enzymes into their prospective meals to begin the digestion process from the inside out, before they even consume the meals.
Snake venom, a highly modified form of saliva, consists of 50 to 60 different protein and peptide components that differ among species of snake and possibly even among individual snakes of the same species. Consider rattlesnakes (Figure 14.11). Rattlesnake venom contains a host of enzymes that digest the tissues of the victim. Phospholipases digest cell membranes at the site of the snakebite, causing a loss of cellular components. The phospholipases also disrupt the membranes of red blood cells, destroying them (a process called hemolysis). Collagenase digests the protein collagen, a major component of connective tissue, whereas hyaluronidase digests hyaluronidate, a glycosaminoglycan component of connective tissue. The combined action of both collagenase and hyaluronidase is to destroy tissue at the site of the bite, enabling the venom to spread more readily throughout the victim.
Various proteolytic enzymes in the venom degrade basement membranes (a thin sheet of fibrous proteins, including collagen, that underlies the epithelial cells) and components of the extracellular matrix, leading to severe tissue damage. Some venoms contain proteolytic enzymes that stimulate the formation of blood clots as well as enzymes that digest blood clots. The net effect of these enzymes acting in concert may be to deplete all clotting factors from the blood, and so clots do not form. Venoms also contain various peptides that have neurotoxic activities. The neurotoxins immobilize the prey, whereas the digestive enzymes reduce the size of the prey to make swallowing easier.
255
Despite the toxic nature of snake venoms, and venoms of all sorts, the study of the components of deadly concoctions have yielded a virtual pharmacopeia of clinically useful drugs. Drugs to combat hypertension (high blood pressure) have been developed following studies of venom components of the South African pit viper Bothrops jararaca. Drugs that reduce the likelihood of myocardial infarction (heart attack) resulted from the examination of the venom of the Southeastern pigmy rattlesnake Sistrurus miliarius barbouri. A component of the venom from the copperhead Agkistrodon acutus is showing promise as an anticancer drug.