16.2 NAD+ Is Regenerated from the Metabolism of Pyruvate
✓ 2 Explain why the regeneration of NAD+ is crucial to fermentations.
The conversion of glucose into two molecules of pyruvate results in the net synthesis of ATP. However, an energy-
292
Fermentations Are a Means of Oxidizing NADH
NUTRITION FACTS

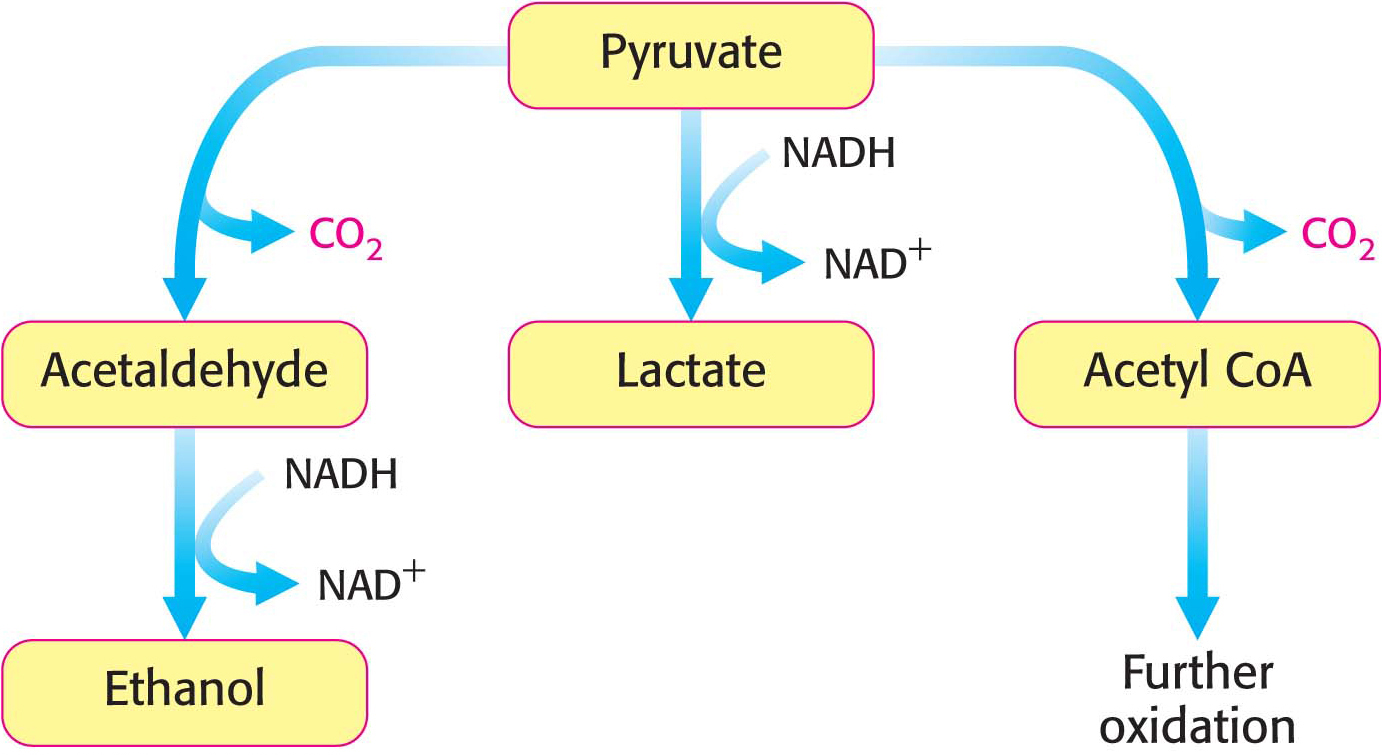
The sequence of reactions from glucose to pyruvate is similar in most organisms and most types of cells. In contrast, the fate of pyruvate is variable. Three reactions of pyruvate are of primary importance: conversion into ethanol, lactate, or carbon dioxide and water (Figure 16.4). The first two reactions are fermentations that take place in the absence of oxygen. Fermentations are ATP-
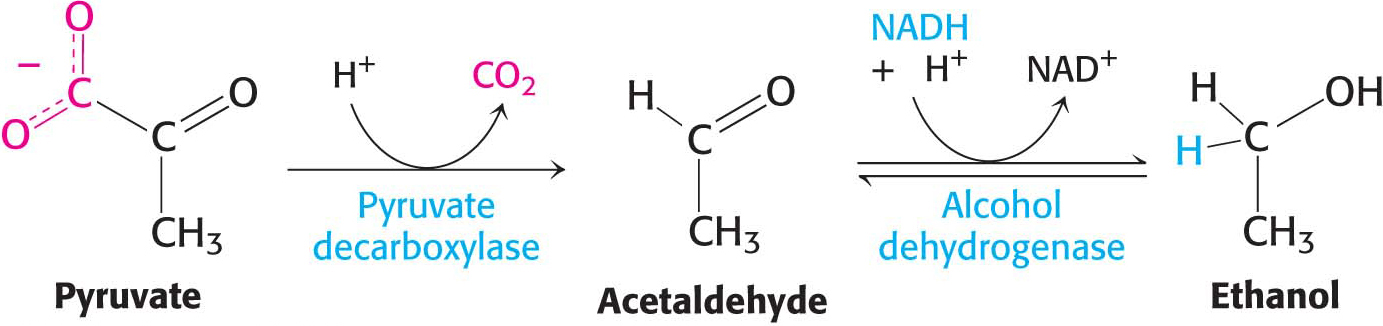
Ethanol is formed from pyruvate in yeast and several other microorganisms. The first step is the decarboxylation of pyruvate. This reaction is catalyzed by pyruvate decarboxylase, which requires the coenzyme thiamine pyrophosphate. This coenzyme is derived from the vitamin thiamine (B1). The second step is the reduction of acetaldehyde to ethanol by NADH, in a reaction catalyzed by alcohol dehydrogenase. Acetaldehyde is thus the organic compound that accepts the electrons in this fermentation. This reaction regenerates NAD+:
The conversion of glucose into ethanol is an example of alcoholic fermentation. The net result of this anaerobic process is
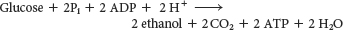
Note that NAD+ and NADH do not appear in this equation, even though they are crucial for the overall process. NADH generated by the oxidation of glyceraldehyde 3-
phosphate is consumed in the reduction of acetaldehyde to ethanol. Thus, there is no net oxidation- reduction in the conversion of glucose into ethanol (Figure 16.5). The ethanol formed in alcoholic fermentation is a key ingredient in brewing and winemaking, and the carbon dioxide formed accounts for some of the carbonation in beer and champagne. 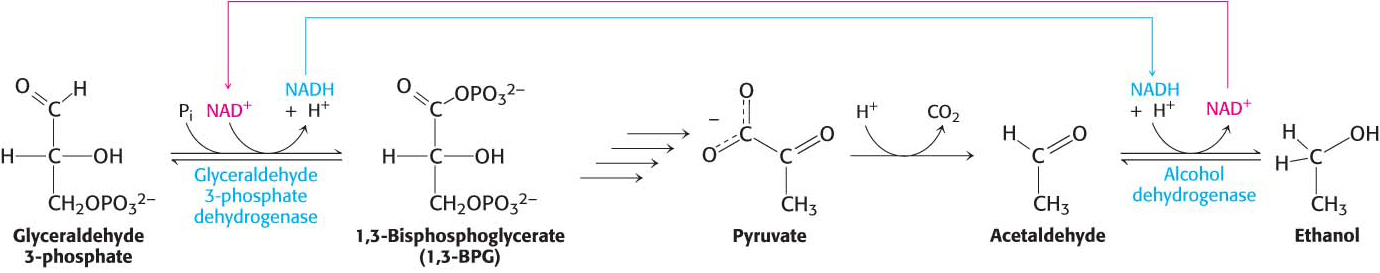 Figure 16.5: Maintaining redox balance in alcoholic fermentation. The NADH produced by the glyceraldehyde 3-
Figure 16.5: Maintaining redox balance in alcoholic fermentation. The NADH produced by the glyceraldehyde 3-phosphate dehydrogenase reaction must be reoxidized to NAD+ for the glycolytic pathway to continue. In alcoholic fermentation, alcohol dehydrogenase oxidizes NADH and generates ethanol. NUTRITION FACTS
 Thiamine Also called vitamin B1, thiamine is a component of the coenzyme thiamine pyrophosphate (TPP), which is used in decarboxylation reactions. Pork and legumes are good sources of thiamine. A deficiency of thiamine results in beriberi, the symptoms of which include muscle weakness, anorexia, and an enlarged heart.
Thiamine Also called vitamin B1, thiamine is a component of the coenzyme thiamine pyrophosphate (TPP), which is used in decarboxylation reactions. Pork and legumes are good sources of thiamine. A deficiency of thiamine results in beriberi, the symptoms of which include muscle weakness, anorexia, and an enlarged heart.293
Lactate is formed from pyruvate in a variety of microorganisms in a process called lactic acid fermentation. Pyruvate accepts the electrons from NADH to form lactate in a reaction catalyzed by lactate dehydrogenase:
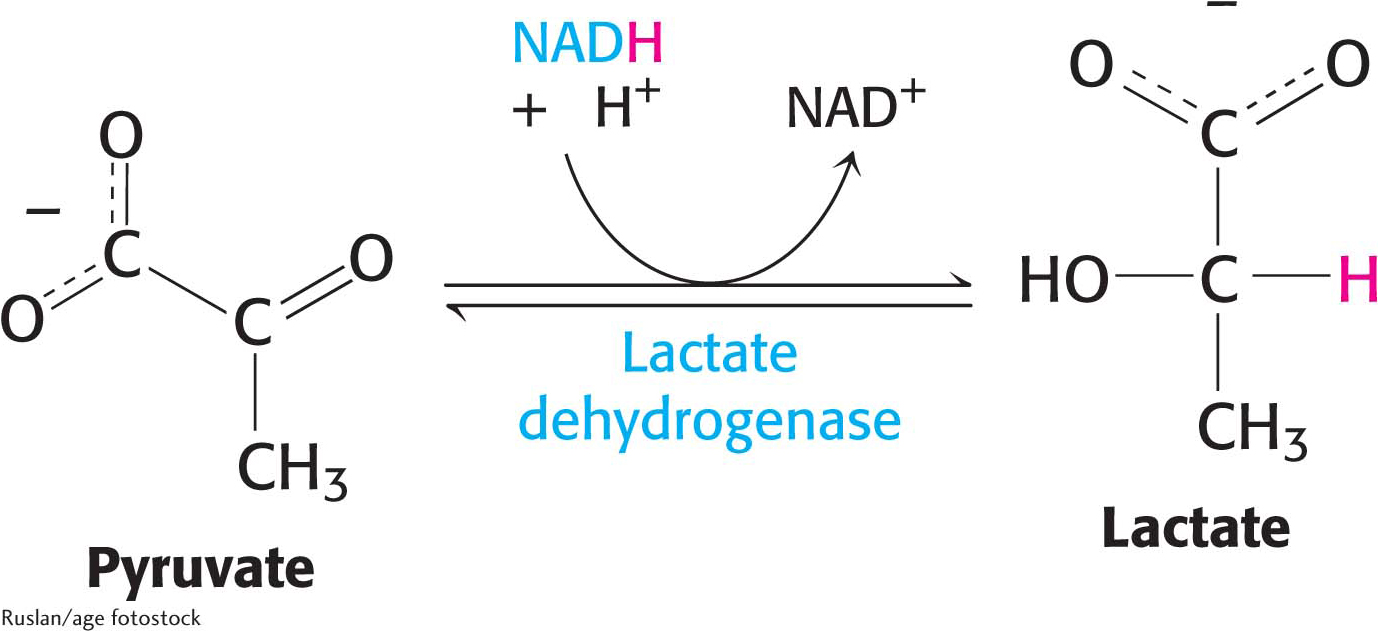
The overall reaction in the conversion of glucose into lactate is
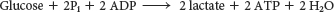
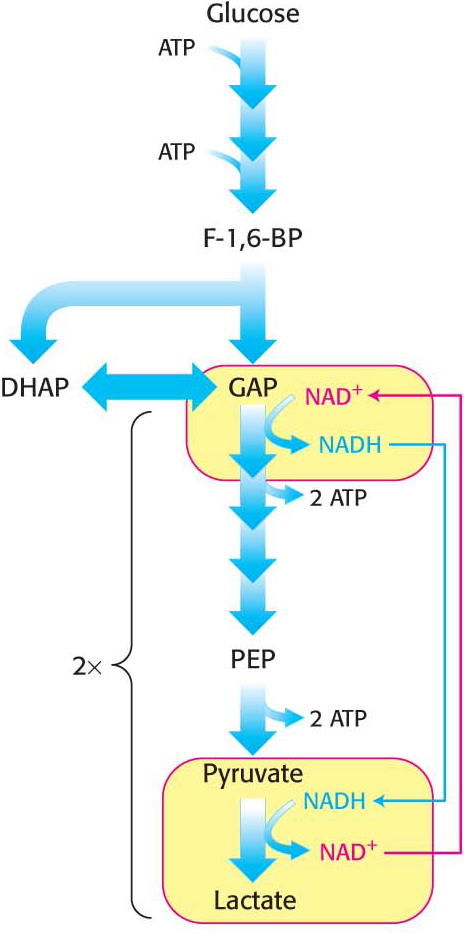 Figure 16.6: Maintaining redox balance in lactic acid fermentation. In lactic acid fermentation, lactate dehydrogenase oxidizes NADH to produce lactic acid and regenerate NAD+.
Figure 16.6: Maintaining redox balance in lactic acid fermentation. In lactic acid fermentation, lactate dehydrogenase oxidizes NADH to produce lactic acid and regenerate NAD+.As in alcoholic fermentation, there is no net oxidation-
reduction. The NADH formed in the oxidation of glyceraldehyde 3- phosphate is consumed in the reduction of pyruvate (Figure 16.6). The regeneration of NAD+ in the reduction of pyruvate to lactate or ethanol sustains the continued process of glycolysis under anaerobic conditions. Certain types of skeletal muscles in most animals can function anaerobically for short periods. For example, a specific type of muscle fiber, called fast twitch or type IIb fibers, perform short bursts of intense exercise. The ATP needs rise faster than the ability of the body to provide oxygen to the muscle. The muscle functions anaerobically until fatigue sets in, which is caused, in part, by lactate buildup. Indeed, the pH of resting type IIb muscle fibers, which is about 7.0, may fall to as low as 6.3 during the bout of exercise. The drop in pH inhibits phosphofructokinase. A lactate/H+ symporter allows the exit of lactate and H+ from the muscle cell.
Only a fraction of the energy of glucose is released in its anaerobic conversion into ethanol or lactate. Much more energy can be extracted aerobically by means of the citric acid cycle and the electron-
transport chain, which combust, or oxidize, glucose all the way to CO2 and H2O. The entry point to this oxidative pathway is acetyl coenzyme A (acetyl CoA), which is formed from pyruvate inside mitochondria: 
This reaction will be considered in detail in Chapter 18. The NAD+ required for this reaction and for the oxidation of glyceraldehyde 3-
phosphate is regenerated in the electron- transport chain in mitochondria (Chapter 20).
!quickquiz! QUICK QUIZ 2
Lactic acid fermentation and alcoholic fermentation are oxidation-
294
DID YOU KNOW?
Fermentation is an ATP-
!bio! BIOLOGICAL INSIGHT: Fermentations Provide Usable Energy in the Absence of Oxygen
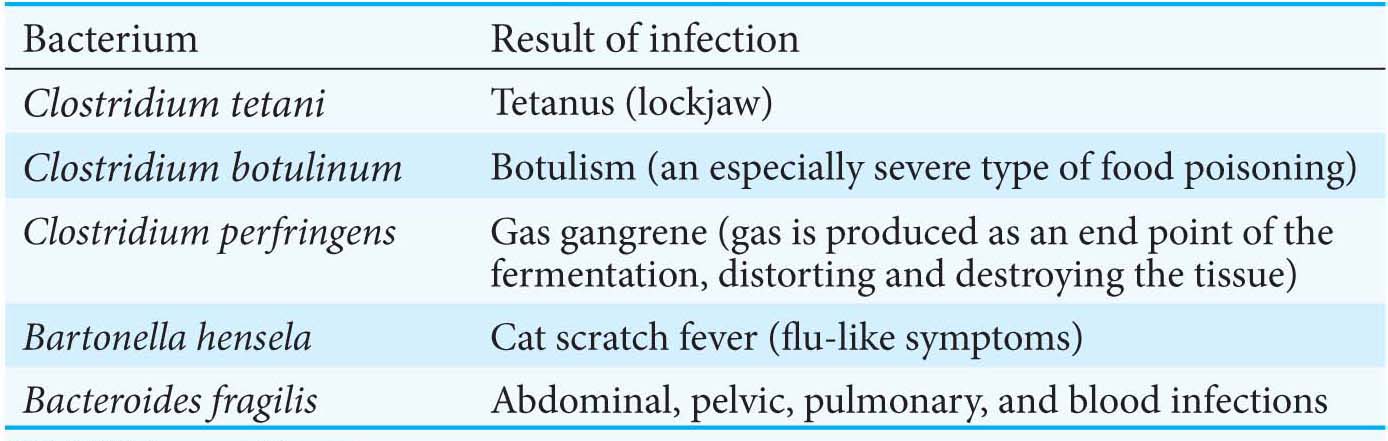
Fermentations yield only a fraction of the energy available from the complete combustion of glucose. Why is a relatively inefficient metabolic pathway so extensively used? The fundamental reason is that fermentations do not require oxygen. The ability to survive without oxygen affords a host of living accommodations such as soils, deep water, and skin pores. Some organisms, called obligate anaerobes, cannot survive in the presence of O2, which is a highly reactive compound. The bacterium Clostridium perfringens, the cause of gangrene, is an example of an obligate anaerobe. Other pathogenic obligate anaerobes are listed in Table 16.2. Some organisms, such as yeast, are facultative anaerobes that metabolize glucose aerobically when oxygen is present and perform fermentation when oxygen is absent.
Many food products, including sour cream, yogurt, various cheeses, beer, wine, and sauerkraut, result from fermentation. Yogurt is produced by the fermentation of lactose in milk to lactate by a mixed culture of Lactobacillus acidophilus and Streptococcus thermophilus. Sour cream begins with pasteurized light cream, which is fermented to lactate by Streptococcus lactis. The lactate is further fermented to ketones and aldehydes by Leuconostoc citrovorum. The second fermentation adds to the taste and aroma of sour cream. Yeast, Saccharomyces cerevisiae, ferments carbohydrates to ethanol and carbon dioxide, providing some of the ingredients for an array of alcohol beverages. Although we have considered only lactic acid and alcoholic fermentation, microorganisms are capable of generating a wide array of molecules as end points to fermentation.