18.1 Pyruvate Dehydrogenase Forms Acetyl Coenzyme A from Pyruvate
✓ 1 Explain why the reaction catalyzed by the pyruvate dehydrogenase complex is a crucial juncture in metabolism.
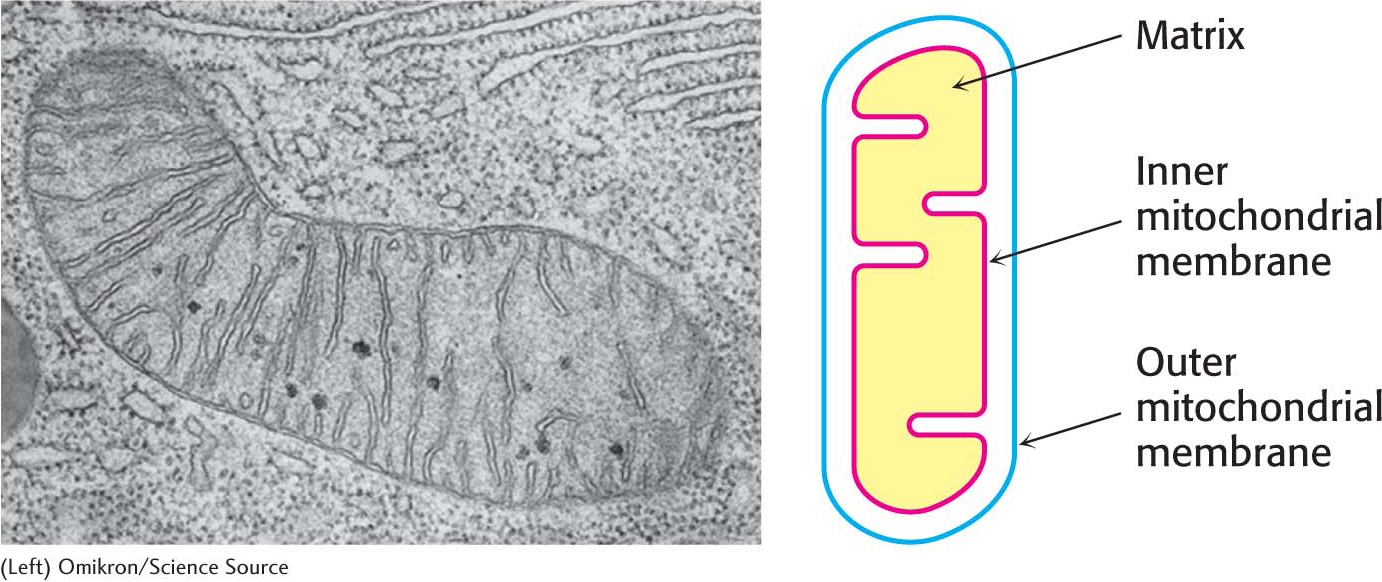
Glycolysis takes place in the cytoplasm of the cell, but the citric acid cycle takes place in mitochondria (Figure 18.3). Pyruvate must therefore be transported into mitochondria to be aerobically metabolized. In the mitochondrial matrix, pyruvate is oxidatively decarboxylated by the pyruvate dehydrogenase complex to form acetyl CoA:
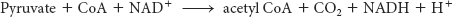
333
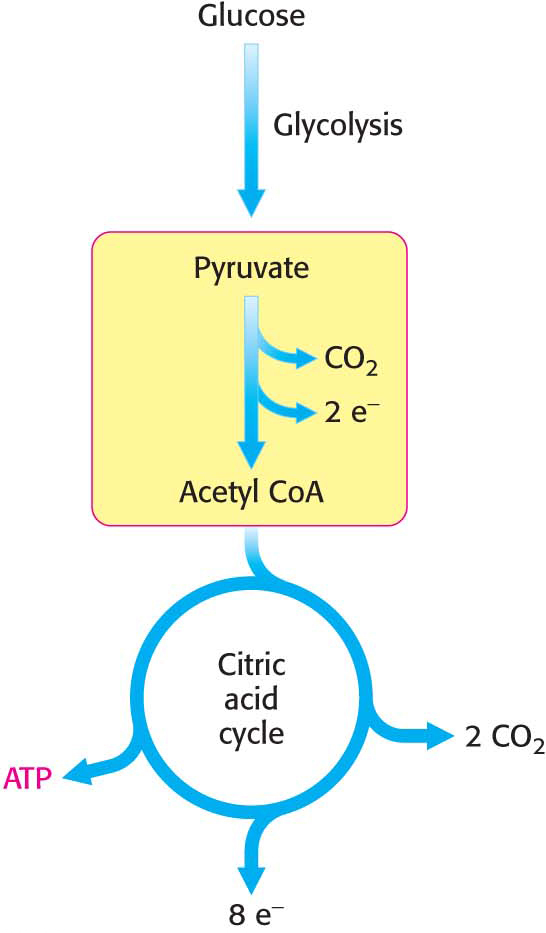
Recall that glycolysis generates two molecules of pyruvate for each glucose molecule metabolized. This irreversible conversion of pyruvate into acetyl CoA is the link between glycolysis and the citric acid cycle (Figure 18.4). This reaction is a decisive reaction in metabolism: it commits the carbon atoms of carbohydrates to oxidation by the citric acid cycle or to the synthesis of lipids (Chapter 29). Note that the pyruvate dehydrogenase complex produces CO2 and captures high-
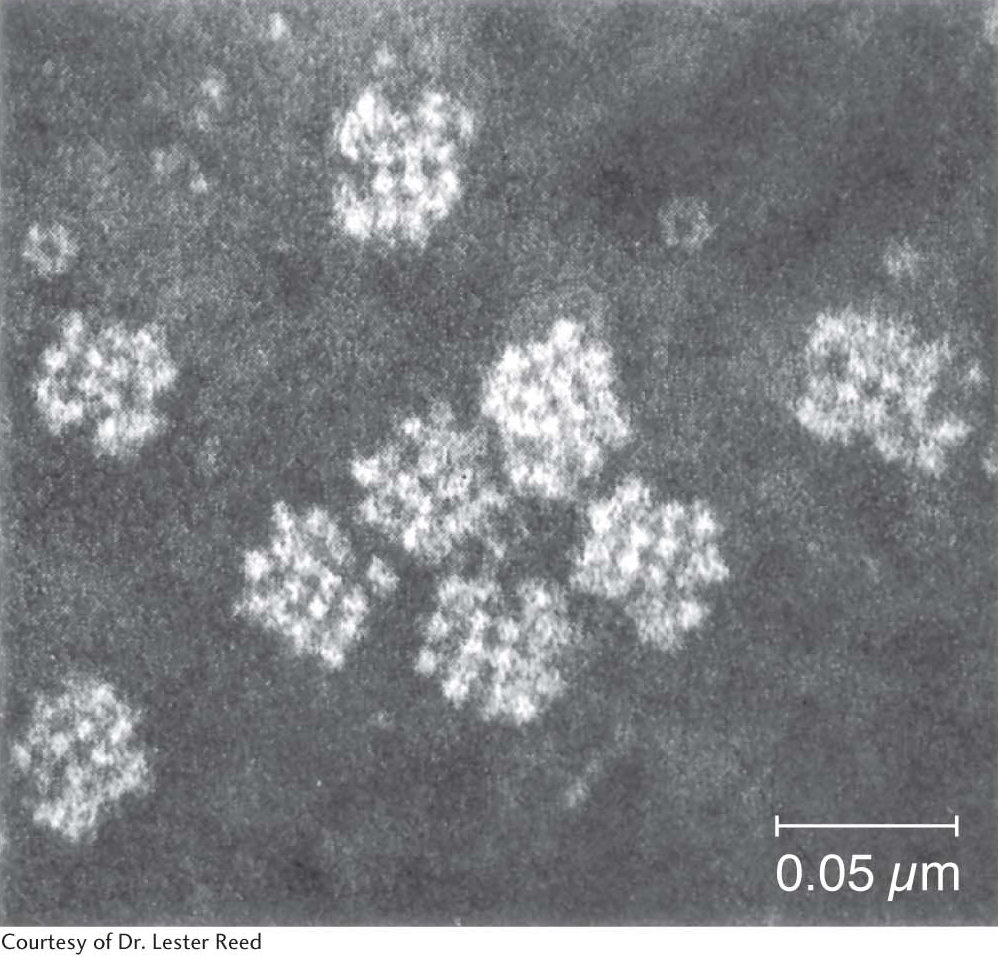
The pyruvate dehydrogenase complex is a large, highly integrated complex of three distinct enzymes (Table 18.1), each with its own active site. It is a member of a family of extremely large similar complexes with molecular masses ranging from 4 million to 10 million daltons (Figure 18.5). As we will see, their elaborate structures allow substrates to travel efficiently from one active site to another, connected by tethers to the core of the complex.
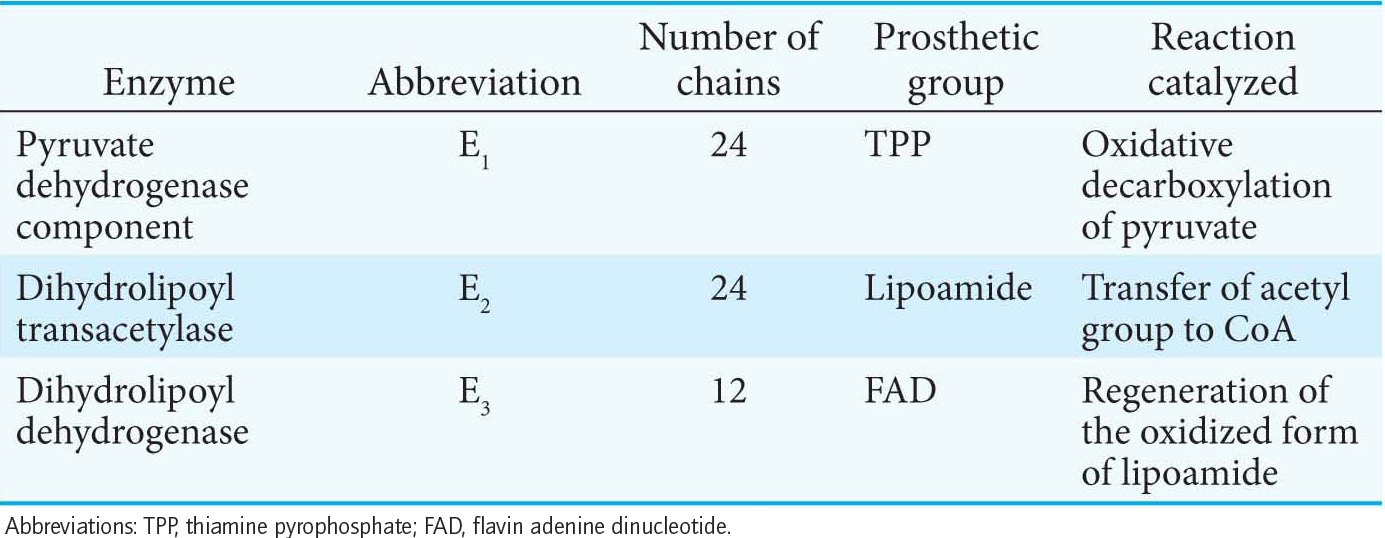
The Synthesis of Acetyl Coenzyme A from Pyruvate Requires Three Enzymes and Five Coenzymes
We will examine the mechanism of action of the pyruvate dehydrogenase complex in some detail because it catalyzes a key step in metabolism—
334
The conversion of pyruvate into acetyl CoA consists of three steps: decarboxylation, oxidation, and the transfer of the resultant acetyl group to CoA:
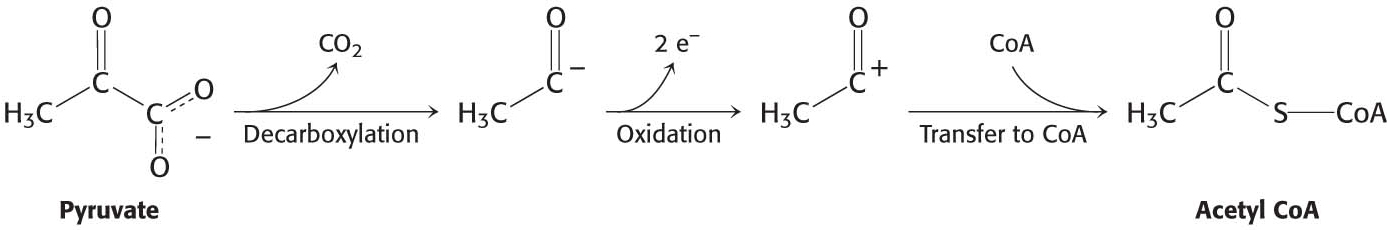
These steps must be coupled to preserve the free energy derived from the decarboxylation step to drive the formation of NADH and acetyl CoA.
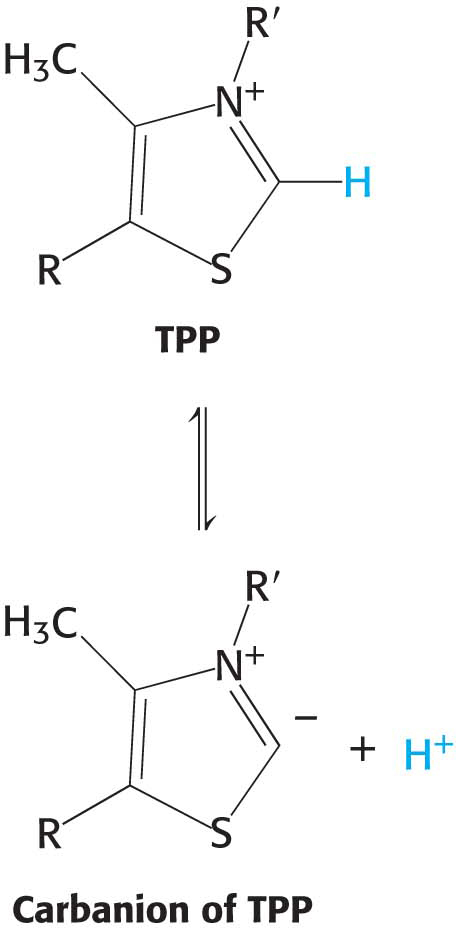
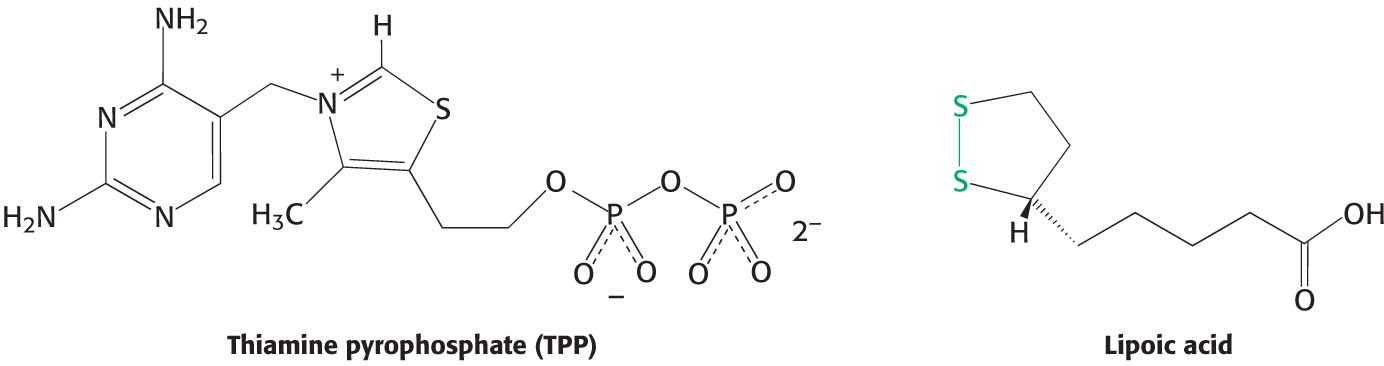
Decarboxylation. Pyruvate combines with the ionized (carbanion) form of TPP and is then decarboxylated to yield hydroxyethyl-
TPP: 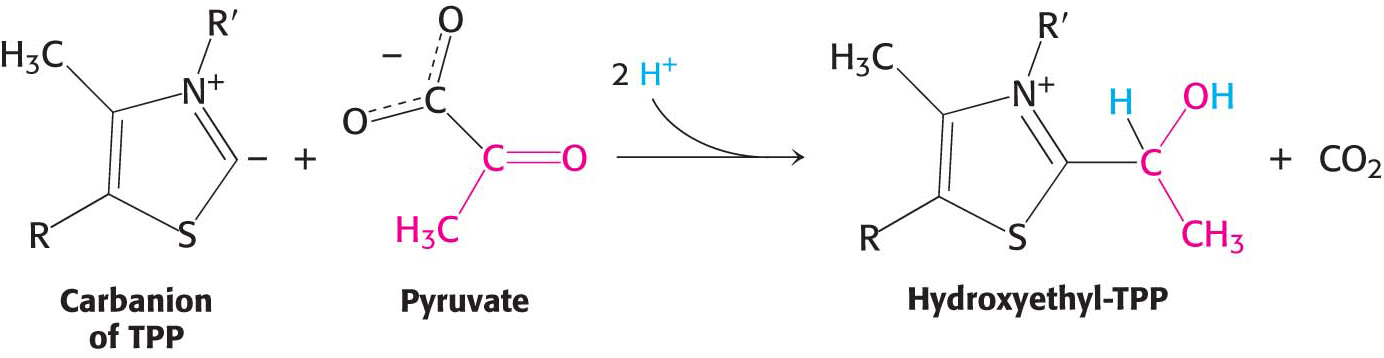
This reaction, the rate-
limiting step in the synthesis of acetyl CoA, is catalyzed by the pyruvate dehydrogenase component (E1) of the multienzyme complex. TPP is the coenzyme of the pyruvate dehydrogenase component. Oxidation. The hydroxyethyl group attached to TPP is oxidized to form an acetyl group while being simultaneously transferred to lipoamide, a derivative of lipoic acid. Note that this transfer results in the formation of an energy-
rich thioester bond. 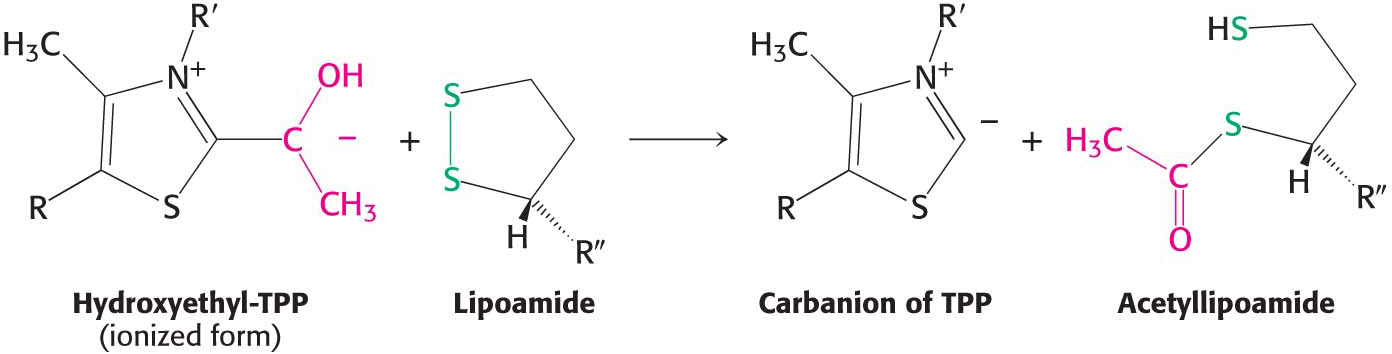
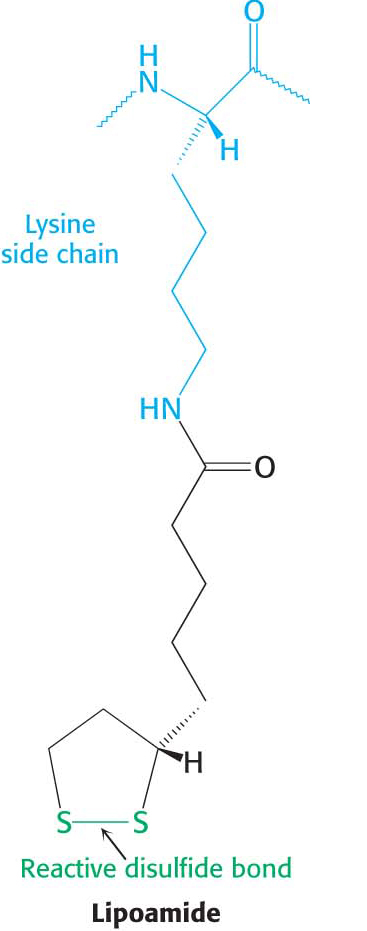
The disulfide group of lipoamide is reduced to its disulfhydryl form in this reaction. The reaction, also catalyzed by the pyruvate dehydrogenase component E1, yields acetyllipoamide.
Formation of acetyl CoA. The acetyl group is transferred from acetyllipoamide to CoA to form acetyl CoA. Dihydrolipoyl transacetylase (E2) catalyzes this reaction. The energy-
rich thioester bond is preserved as the acetyl group is transferred to CoA. Acetyl CoA, the fuel for the citric acid cycle, has now been generated from pyruvate: 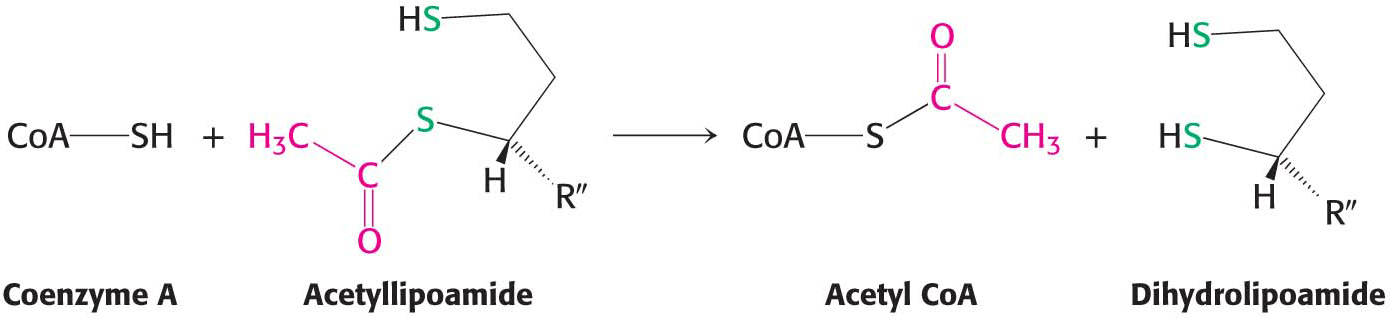
335
However, the pyruvate dehydrogenase complex cannot complete another catalytic cycle until the dihydrolipoamide is oxidized to lipoamide. In a fourth step, the oxidized form of lipoamide is regenerated by dihydrolipoyl dehydrogenase (E3). Two electrons are transferred to an FAD prosthetic group of the enzyme and then to NAD+:
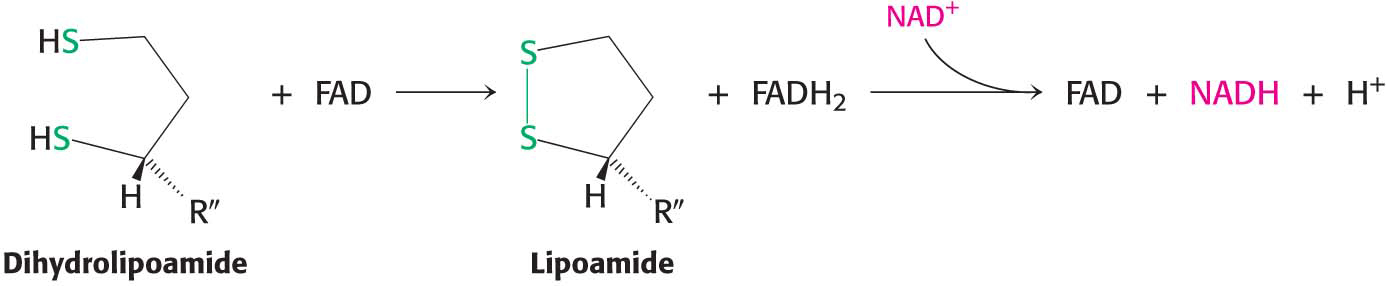
This electron transfer from FAD to NAD+ is unusual because the common role for FAD is to receive electrons from NADH. The electron-
Flexible Linkages Allow Lipoamide to Move Between Different Active Sites
The structures of all of the component enzymes of the pyruvate dehydrogenase complex are known, albeit from different complexes and species. Thus, it is now possible to construct an atomic model of the complex to understand its activity.
The core of the complex is formed by the transacetylase component E2. Transacetylase consists of eight catalytic trimers assembled to form a hollow cube. Each of the three subunits forming a trimer has three major domains (Figure 18.6). At the amino terminus is a small domain that contains a flexible lipoamide cofactor covalently attached to a lysine side chain. The lipoamide domain is followed by a small domain that interacts with E3 within the complex. A larger transacetylase domain completes an E2 subunit. The eight E2 trimers constitute the core of the complex and are surrounded by 24 copies of E1 (an α2 β2 tetramer) and 12 copies of E3 (an αβ dimer). In mammals, this core contains another protein, E3-binding protein (E3-BP), which facilitates the interaction between E2 and E3. If E3-BP is missing, the PDH complex has greatly reduced activity.
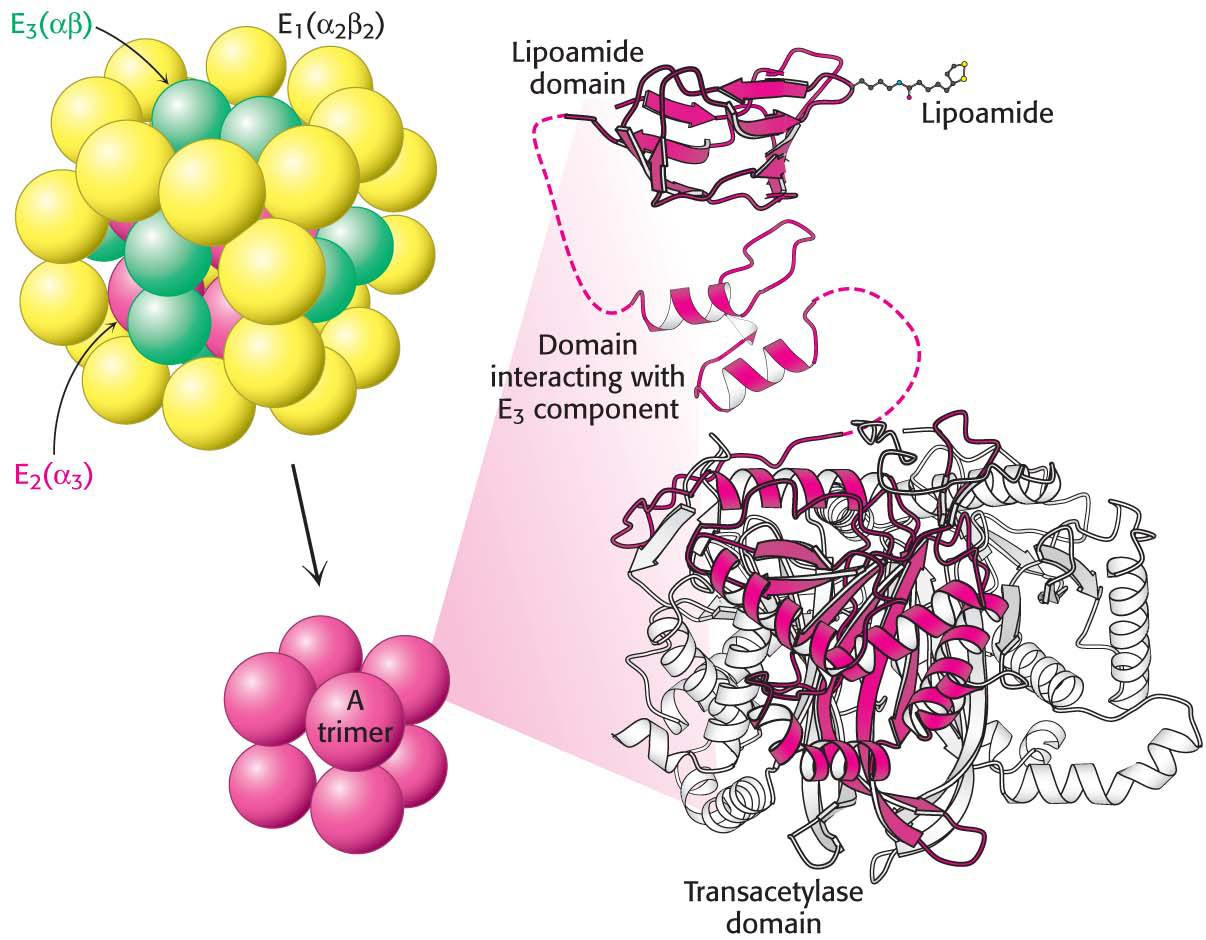
336
How do the three distinct active sites work in concert? The key is the long, flexible lipoamide arm of the E2 subunit, which carries substrate from active site to active site (Figure 18.7).
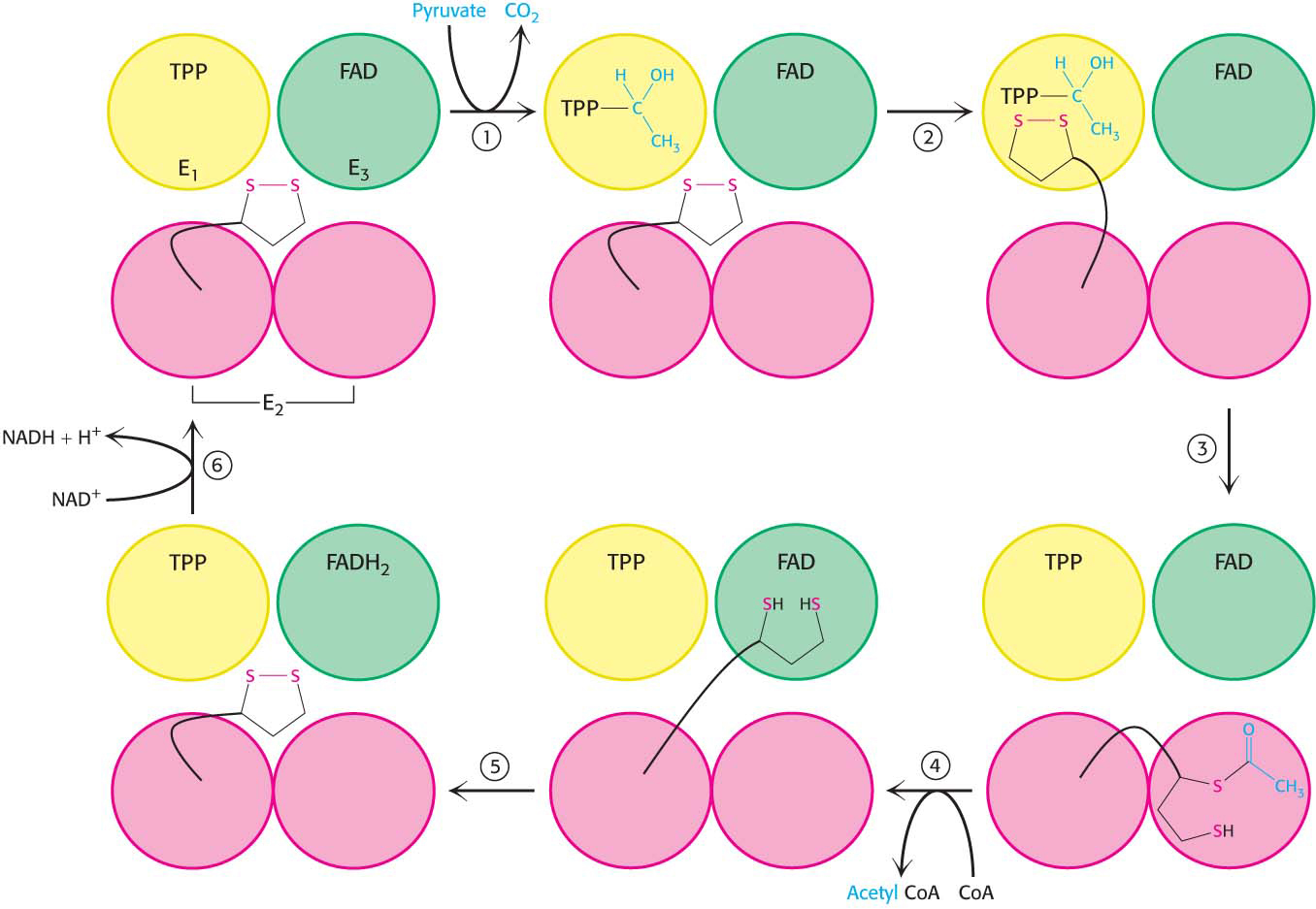
Pyruvate is decarboxylated at the active site of E1, forming the hydroxyethyl-
TPP intermediate, and CO2 leaves as the first product. This active site lies deep within the E1 complex, connected to the enzyme surface by a 20- Å-long hydrophobic channel. E2 inserts the lipoamide arm of the lipoamide domain into the deep channel in E1 leading to the active site.
E1 catalyzes the transfer of the acetyl group to the lipoamide. The acetylated arm then leaves E1 and enters the E2 cube to visit the active site of E2, located deep in the cube at the subunit interface.
The acetyl moiety is transferred to CoA, and the second product, acetyl CoA, leaves the cube. The reduced lipoamide arm then swings to the active site of the E3 flavoprotein.
337
At the E3 active site, the lipoamide is oxidized by coenzyme FAD. The reactivated lipoamide is ready to begin another reaction cycle.
The final product, NADH, is produced with the reoxidation of FADH2 to FAD.
The structural integration of three kinds of enzymes and the long flexible lipoamide arm make the coordinated catalysis of a complex reaction possible. The proximity of one enzyme to another increases the overall reaction rate and minimizes side reactions. All the intermediates in the oxidative decarboxylation of pyruvate remain bound to the complex throughout the reaction sequence and are readily transferred as the flexible arm of E2 calls on each active site in turn.