21.3 Cellular Respiration is Regulated by the Need for ATP
✓ 4 Identify the ultimate determinant of the rate of cellular respiration.
We have observed many times that most catabolic pathways are regulated in some fashion by the ATP concentration. Because ATP is the end product of cellular respiration, the ATP needs of the cell are the ultimate determinant of the rate of respiratory pathways and their components. Before we explore the nature of this regulation, let us calculate the ATP yield from the conversion of glucose to CO2 and H2O.
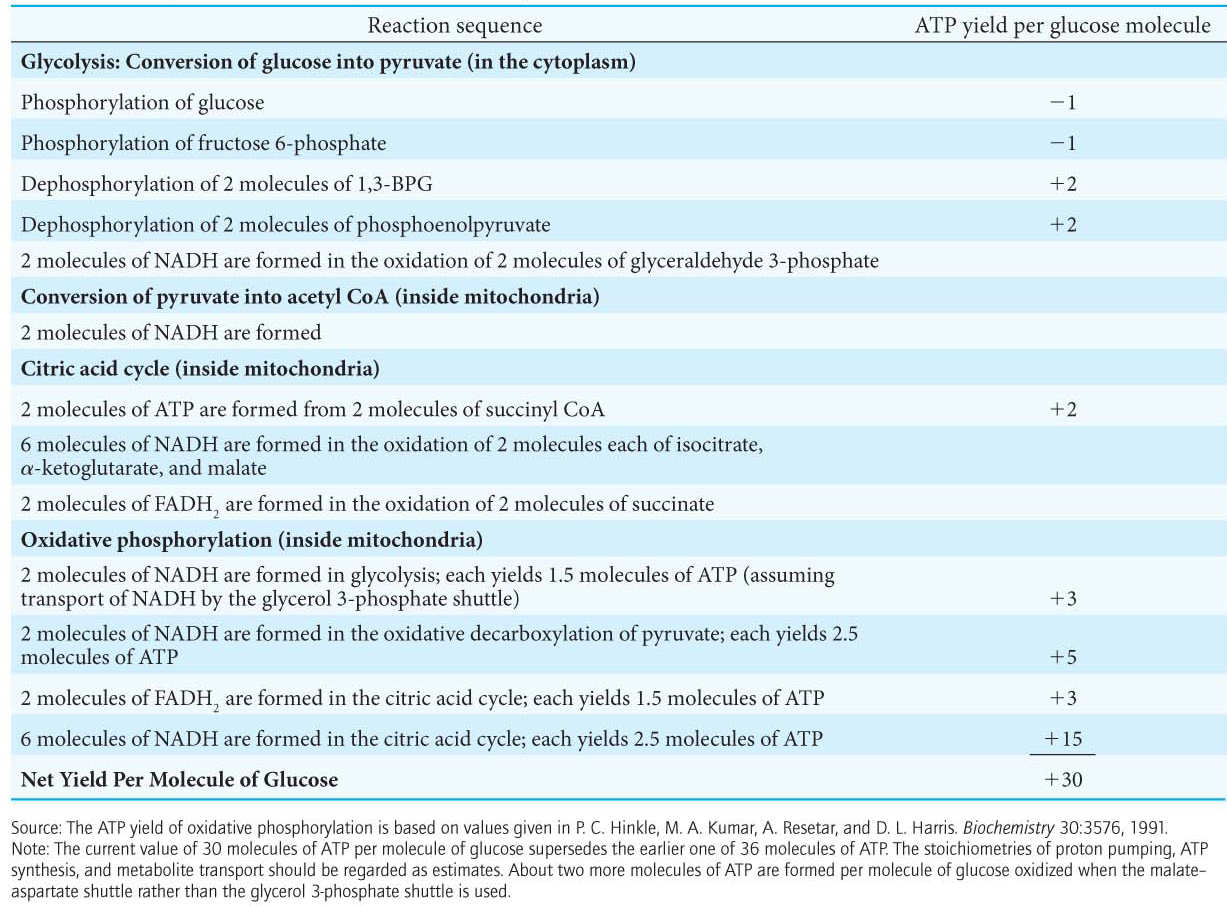
The Complete Oxidation of Glucose Yields About 30 Molecules of ATP
We can estimate how many molecules of ATP are formed when glucose is completely oxidized to CO2. We say “estimate” because, in contrast with the ATP yield of glycolysis and the citric acid cycle (which yield 4 molecules of ATP per molecule of glucose and 1 molecule of ATP per molecule of pyruvate, respectively), the stoichiometries of proton pumping, ATP synthesis, and metabolite-
394
395
The Rate of Oxidative Phosphorylation Is Determined by the Need for ATP
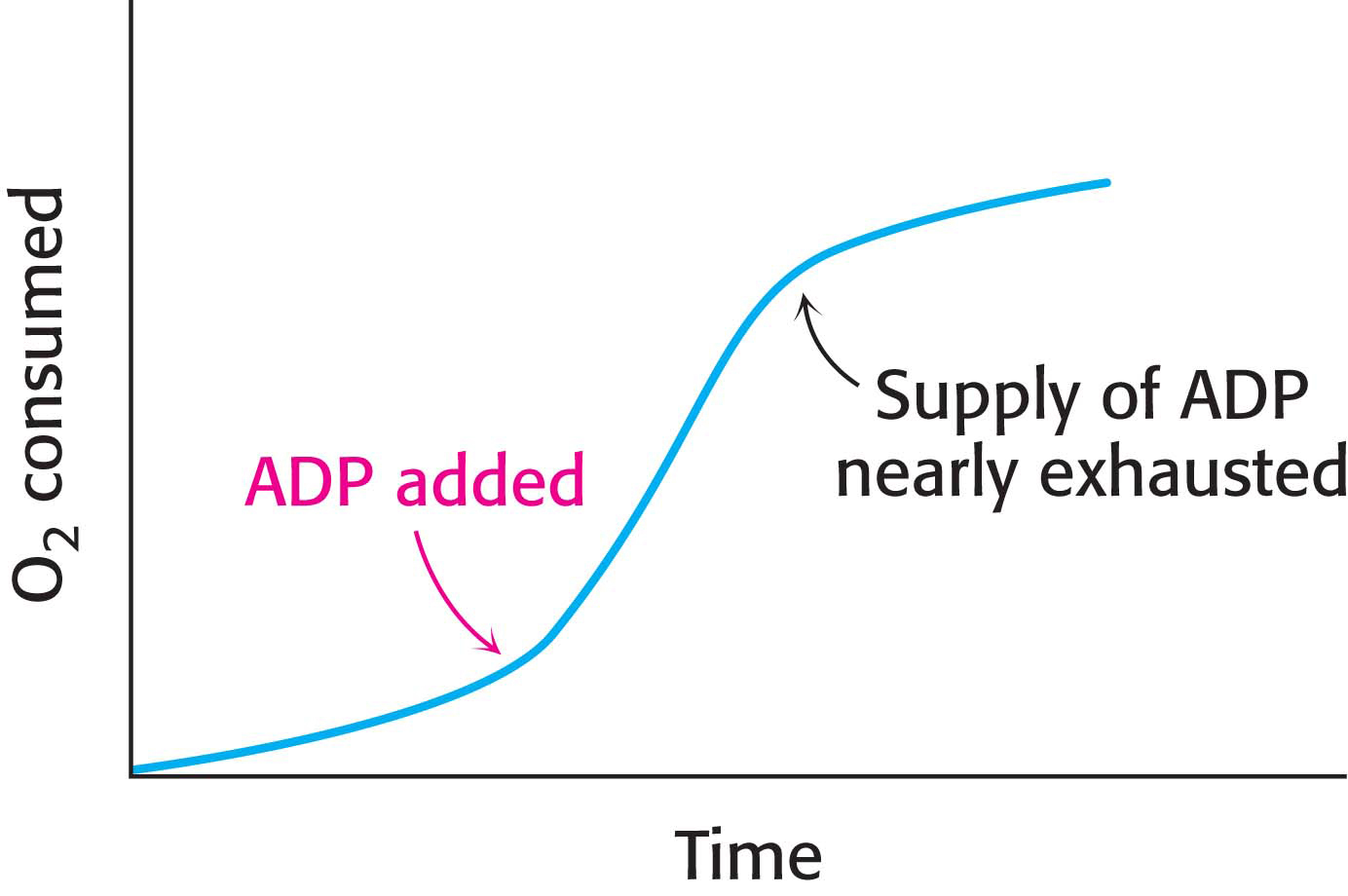
How is the rate of the electron-
As discussed in Chapter 19, the level of ADP likewise indirectly affects the rate of the citric acid cycle. At low concentrations of ADP, as in a resting muscle, NADH and FADH2 produced by the citric acid cycle are not oxidized back to NAD+ and FAD by the electron-
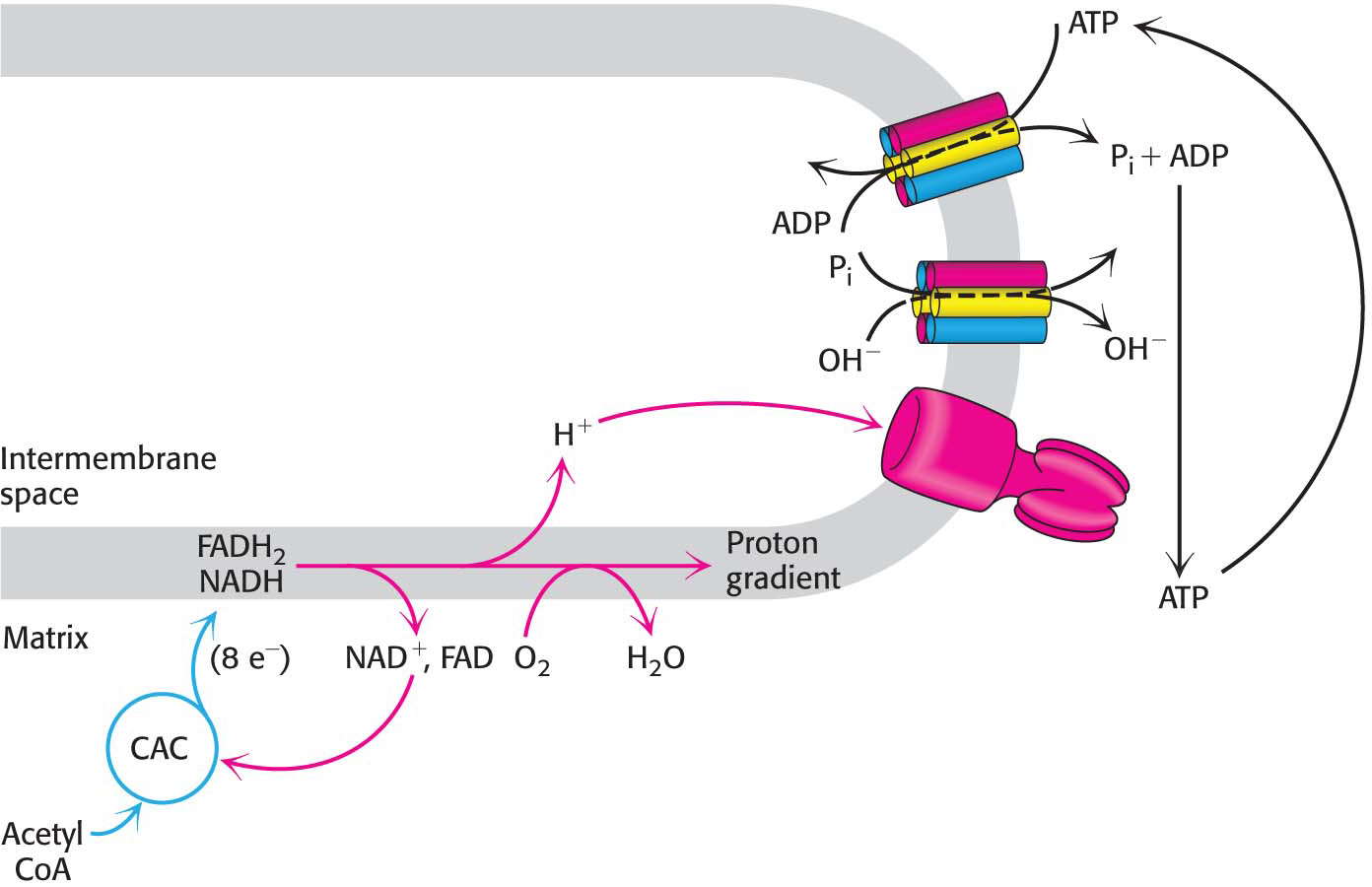
!clinic! CLINICAL INSIGHT: ATP Synthase Can Be Regulated
Mitochondria contain an evolutionarily conserved protein, inhibitory factor 1 (IF1), that specifically inhibits the potential hydrolytic activity of the F0F1 ATP synthase. What is the function of IF1? Consider a circumstance where tissues may be deprived of oxygen (ischemia). Without oxygen as the electron acceptor, the electron transport chain will be unable to generate the proton-
396
IF1 is over-
!bio! BIOLOGICAL INSIGHT: Regulated Uncoupling Leads to the Generation of Heat
Some organisms possess the ability to uncouple oxidative phosphorylation from ATP synthesis to generate heat. Such uncoupling is a means to maintain body temperature in hibernating animals, in some newborn animals (including human beings), and in mammals adapted to cold. In animals, brown fat or brown adipose tissue (BAT) is specialized tissue for this process of nonshivering thermogenesis. In contrast, white adipose tissue (WAT), which constitutes the bulk of adipose tissue, plays no role in thermogenesis but serves as an energy source.
Brown adipose tissue is very rich in mitochondria, often called brown-
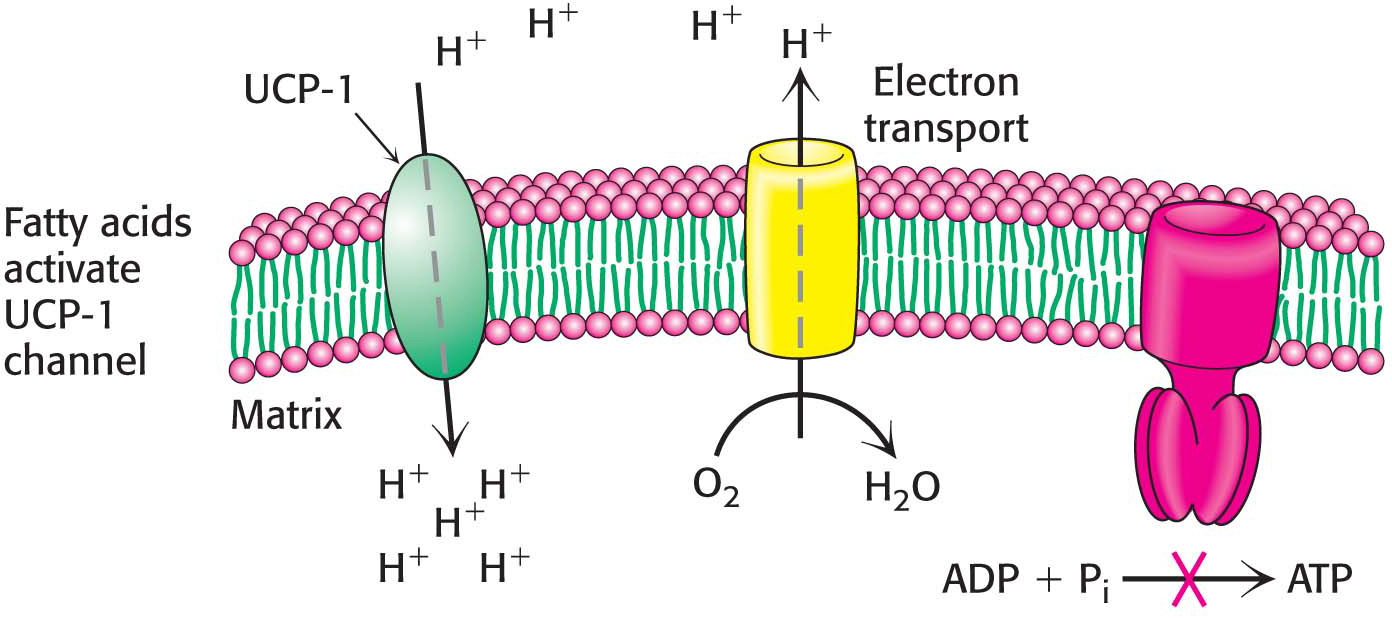
397
We can witness the effects of a lack of nonshivering thermogenesis by examining pig behavior. Pigs are unusual mammals in that they have large litters and are the only ungulates (hoofed animals) that build nests for birth (Figure 21.19). These behavioral characteristics appear to be the result of a biochemical deficiency. Pigs lack UCP-

Until recently, adult humans were believed to lack brown fat tissue. However, new studies have established that adults, women especially, have brown adipose tissue on the neck and upper chest regions that is activated by cold (Figure 21.20). Obesity leads to a decrease in brown adipose tissue.
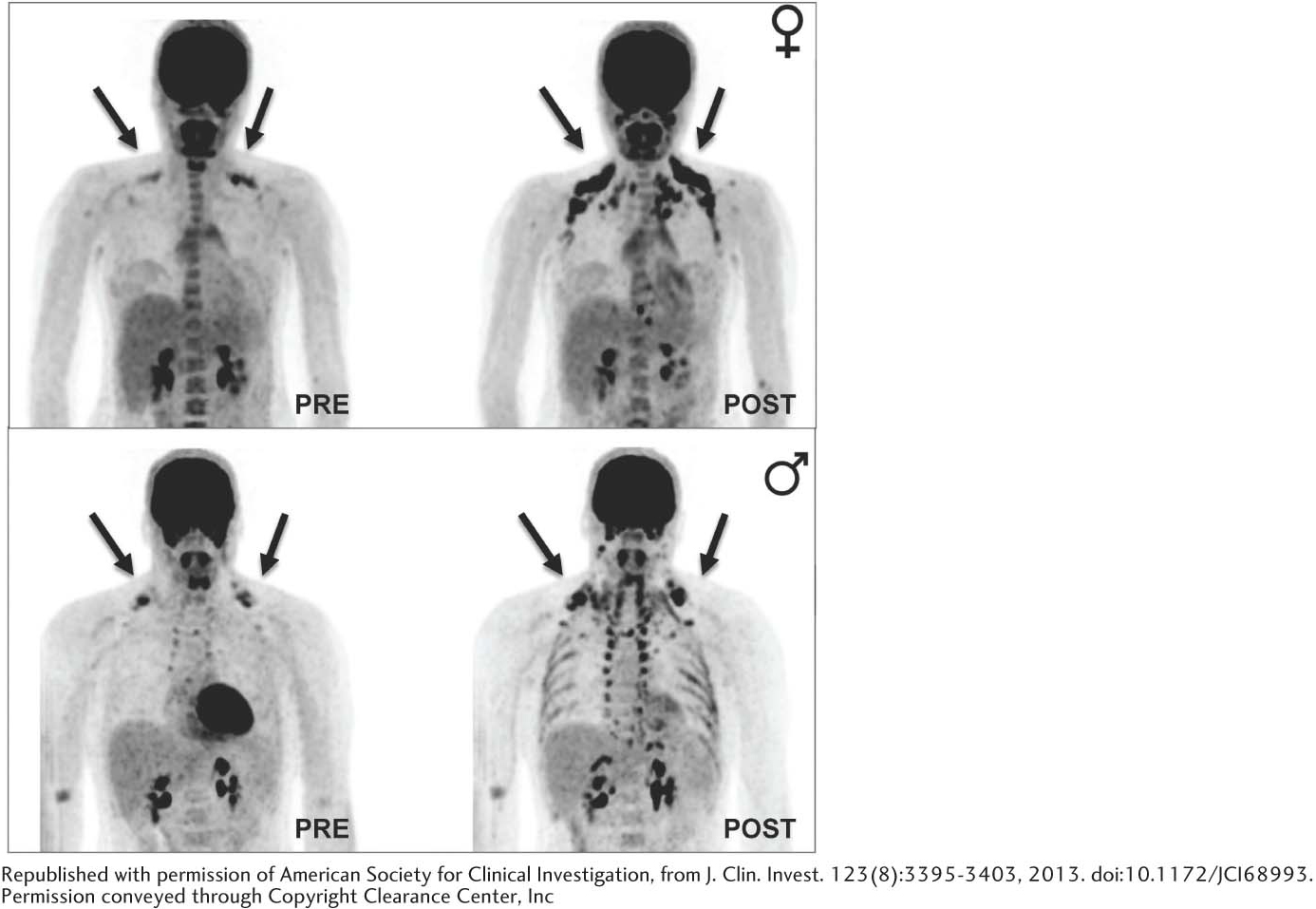
398
!clinic! CLINICAL INSIGHT: Oxidative Phosphorylation Can Be Inhibited at Many Stages
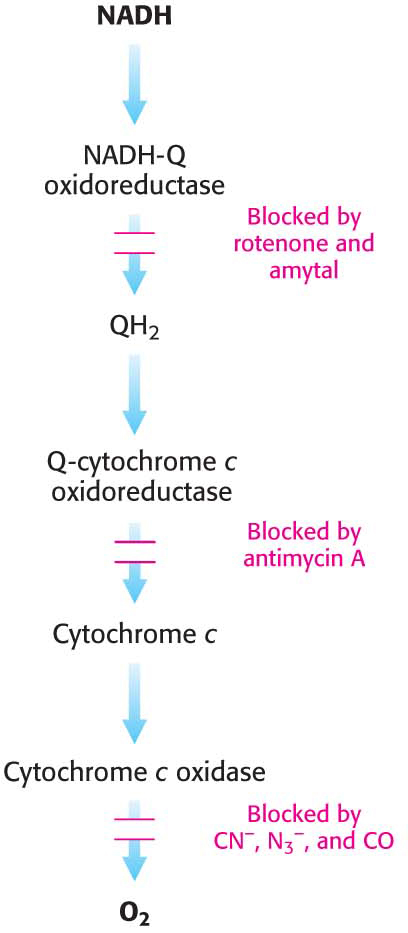
Many potent and lethal poisons exert their effects by inhibiting oxidative phosphorylation at one of a number of different locations.
Inhibition of the electron-
Inhibition of ATP synthase Oligomycin, an antibiotic used as an antifungal agent, and dicyclohexylcarbodiimide (DCC), used in peptide synthesis in the laboratory, prevent the influx of protons through ATP synthase by binding to the carboxylate group of the c subunits required for proton binding. Modification of only one c subunit by DCC is sufficient to inhibit the rotation of the entire c ring and hence ATP synthesis. If actively respiring mitochondria are exposed to an inhibitor of ATP synthase, the electron-
399
Uncoupling electron transport from ATP synthesis The tight coupling of electron transport and phosphorylation in mitochondria can be uncoupled by 2,4-
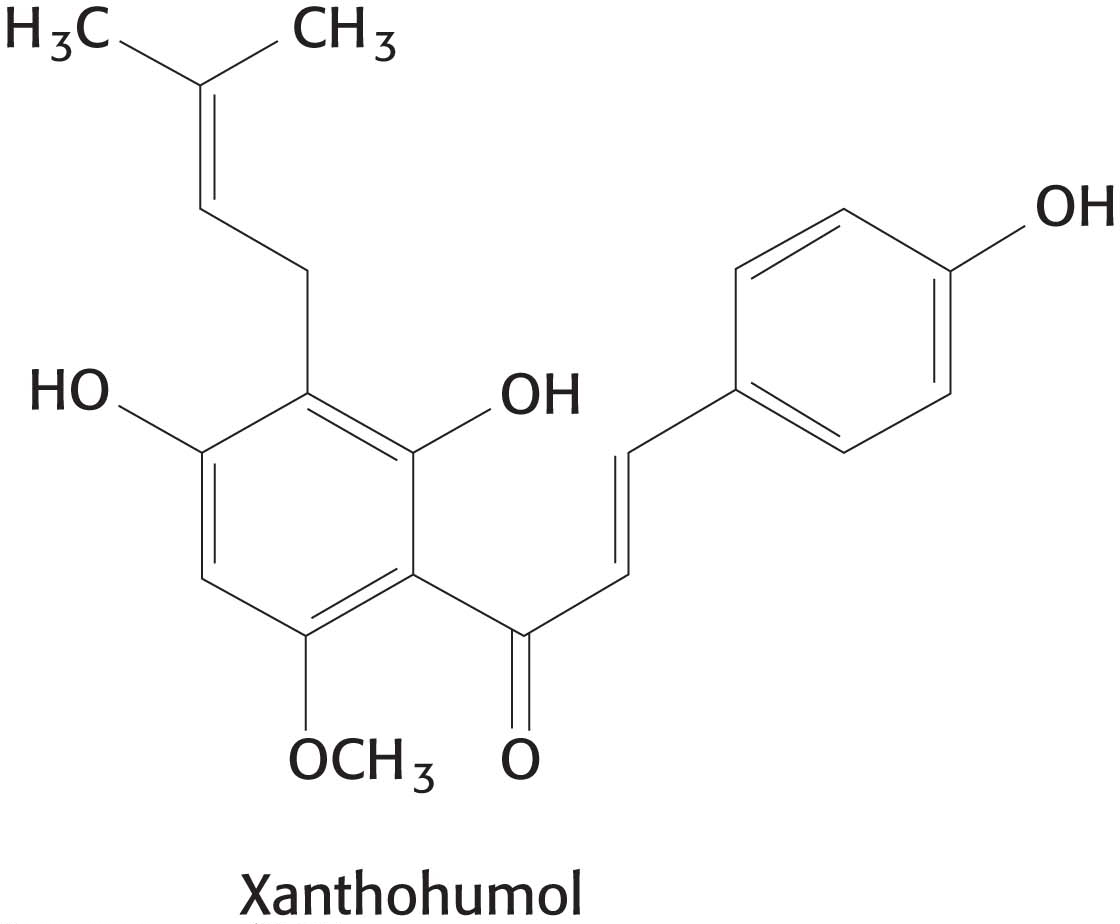
Drugs are being sought that would function as mild uncouplers, uncouplers not as potentially-
Inhibition of ATP export ATP-
!clinic! CLINICAL INSIGHT: Mitochondrial Diseases Are Being Discovered in Increasing Numbers
The number of diseases that can be attributed to mitochondrial mutations is steadily growing in step with our growing understanding of the biochemistry and genetics of mitochondria. Mitochondrial diseases are estimated to affect from 10 to 15 per 100,000 people, roughly equivalent to the prevalence of the muscular dystrophies. The first mitochondrial disease to be understood was Leber hereditary optic neuropathy (LHON), a form of blindness that strikes in midlife as a result of mutations in Complex I. Some of these mutations impair NADH utilization, whereas others block electron transfer to Q. Mutations in Complex I are the most frequent cause of mitochondrial diseases. The accumulation of mutations in mitochondrial genes in a span of several decades may contribute to aging, degenerative disorders, and cancer.
A human egg harbors several hundred thousand molecules of mitochondrial DNA, whereas a sperm contributes only a few hundred and thus has little effect on the mitochondrial genotype. Because the maternally-
400
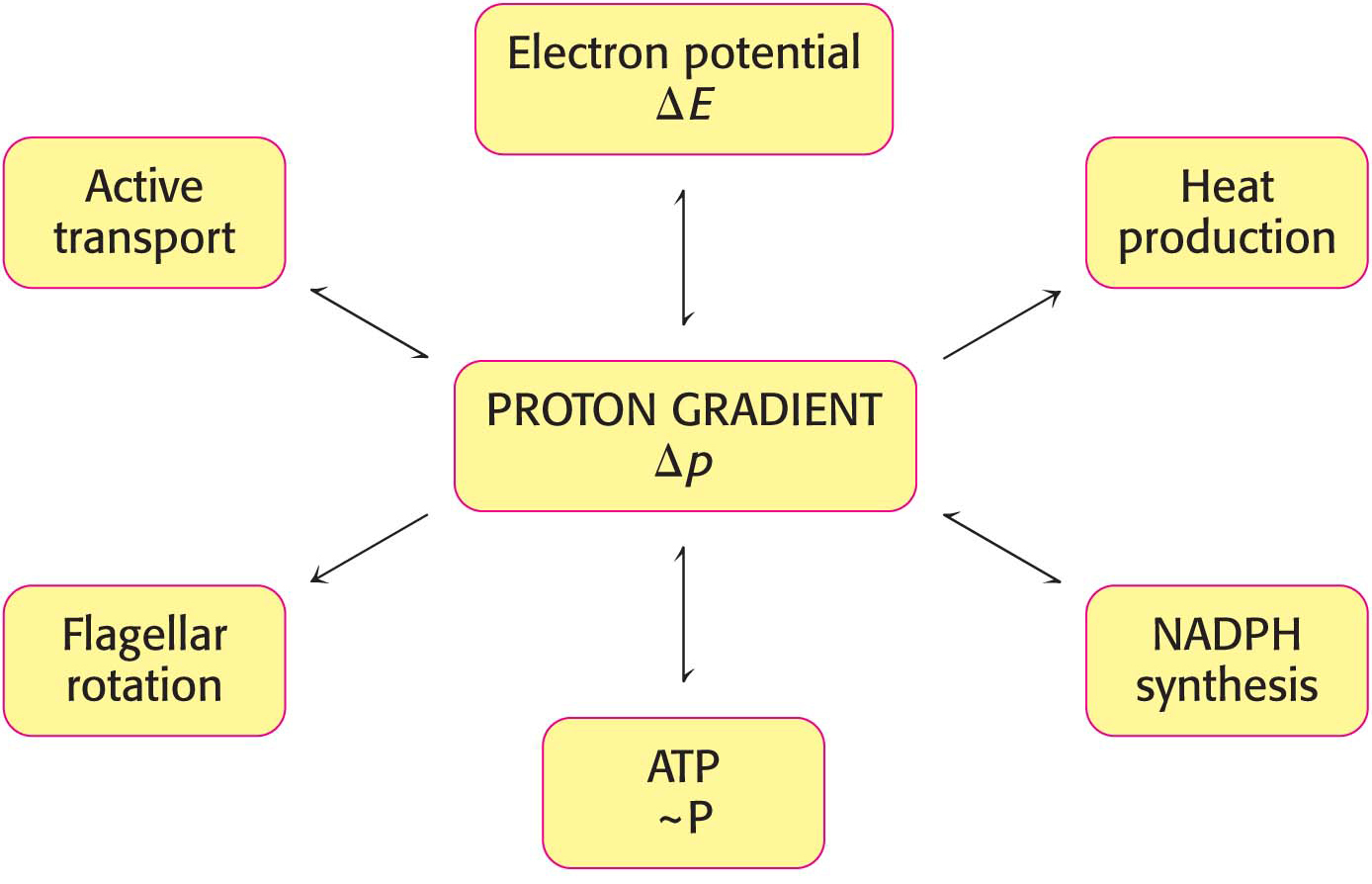
Power Transmission by Proton Gradients Is a Central Motif of Bioenergetics
The main concept presented in this chapter is that mitochondrial electron transfer and ATP synthesis are linked by a transmembrane proton gradient. ATP synthesis in bacteria and chloroplasts also is driven by proton gradients. In fact, proton gradients power a variety of energy-