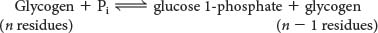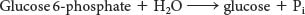Phosphorylase Cleaves Glycogen to Release Glucose 1-phosphate
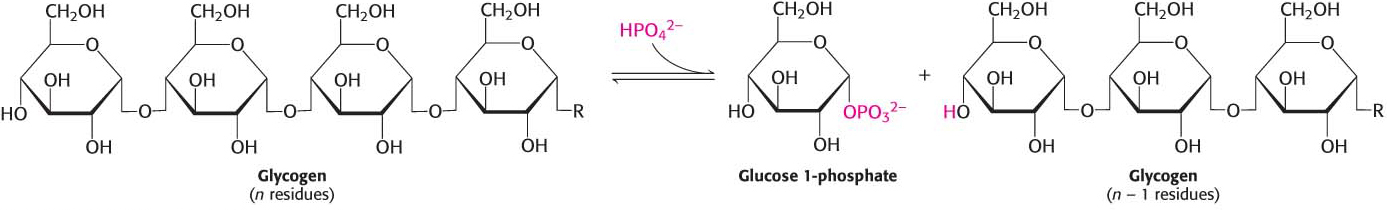
Glycogen phosphorylase, the key regulatory enzyme in glycogen breakdown, cleaves its substrate by the addition of orthophosphate (Pi) to yield glucose 1-phosphate. The cleavage of a bond by the addition of orthophosphate is referred to as phosphorolysis:
Phosphorylase catalyzes the sequential removal of glucosyl residues from the nonreducing ends of the glycogen molecule, as illustrated in Figure 24.2 (the ends with a free OH group on carbon 4). Orthophosphate splits the glycosidic linkage between C-1 of the terminal residue and C-4 of the adjacent one. Specifically, it cleaves the bond between the C-1 carbon atom and the glycosidic oxygen atom, and the a configuration at C-1 of the newly released glucose 1-phosphate is retained:
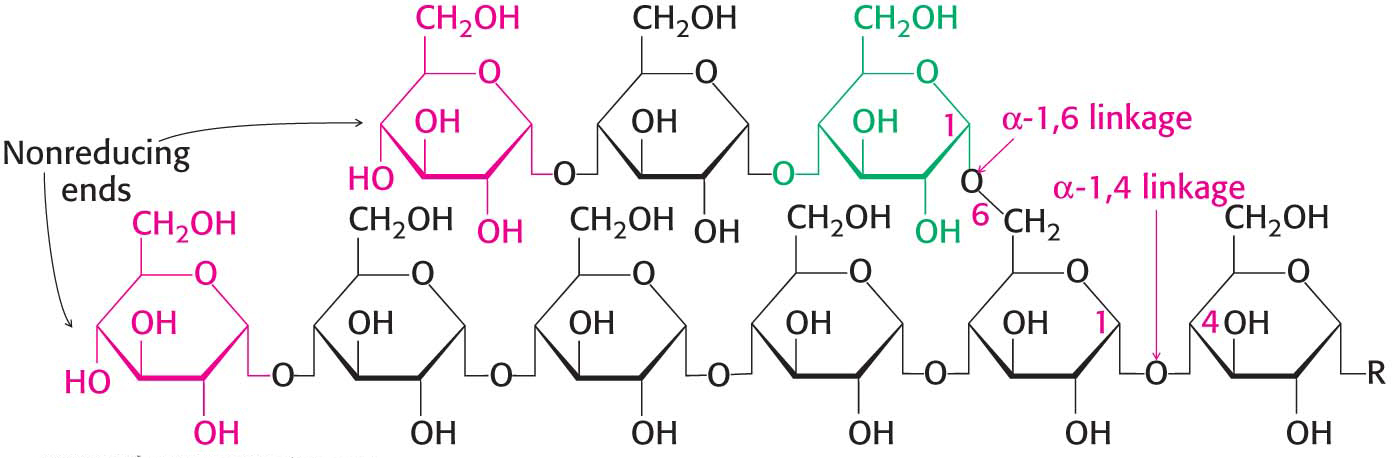
Figure 24.2: Glycogen structure. In this structure of two outer branches of a glycogen molecule, the residues at the nonreducing ends are shown in red and the residue that starts a branch is shown in green. The rest of the glycogen molecule is represented by R.
Glucose 1-phosphate released from glycogen can be readily converted into glucose 6-phosphate, an important metabolic intermediate, by the enzyme phosphoglucomutase.
The phosphorolytic cleavage of glycogen is energetically advantageous because the released sugar is already phosphorylated. In contrast, a hydrolytic cleavage would yield glucose, which would then have to be phosphorylated at the expense of a molecule of ATP to enter the glycolytic pathway. An additional advantage of phosphorolytic cleavage for muscle cells is that no transporters exist for glucose 1-phosphate, which would be negatively charged under physiological conditions, and so it cannot be transported out of the cell.
A Debranching Enzyme Also Is Needed for the Breakdown of Glycogen
Glycogen phosphorylase can carry out the process of degrading glycogen by itself only to a limited extent before encountering an obstacle. The α-1,6-glycosidic bonds at the branch points are not susceptible to cleavage by phosphorylase. Indeed, phosphorylase stops cleaving α-1,4 linkages when it reaches a residue four residues away from a branch point. Because about 1 in 10 residues is branched, glycogen degradation by the phosphorylase alone would halt after the release of six glucose molecules per branch.
How can the remainder of the glycogen molecule be degraded for use as a fuel? Two additional enzymes, a transferase and α-1,6-glucosidase, remodel the glycogen for continued degradation by the phosphorylase. The transferase shifts a block of three glucosyl residues from one outer branch to another. (Figure 24.3). This transfer exposes a single glucose residue joined by an α-1,6-glycosidic linkage. α-1,6-Glucosidase, also known as the debranching enzyme, then hydrolyzes the α-1,6-glycosidic bond, resulting in the release of a free glucose molecule:
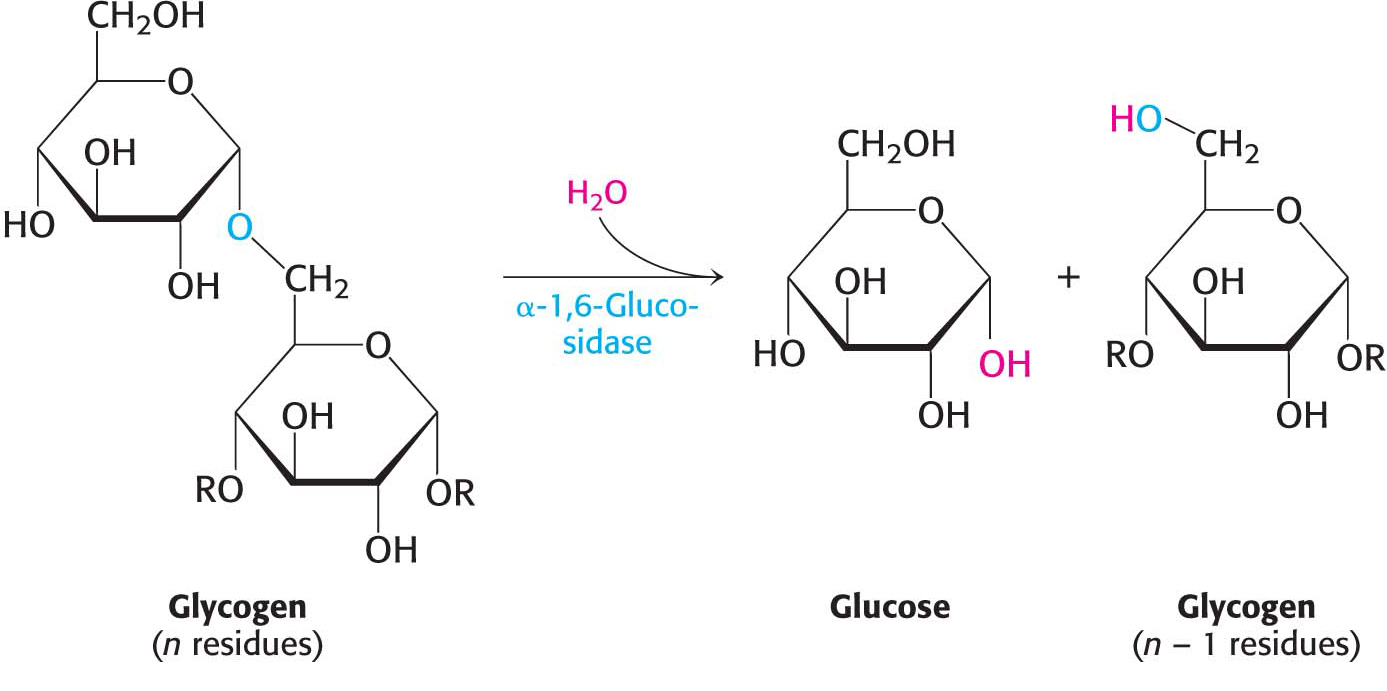
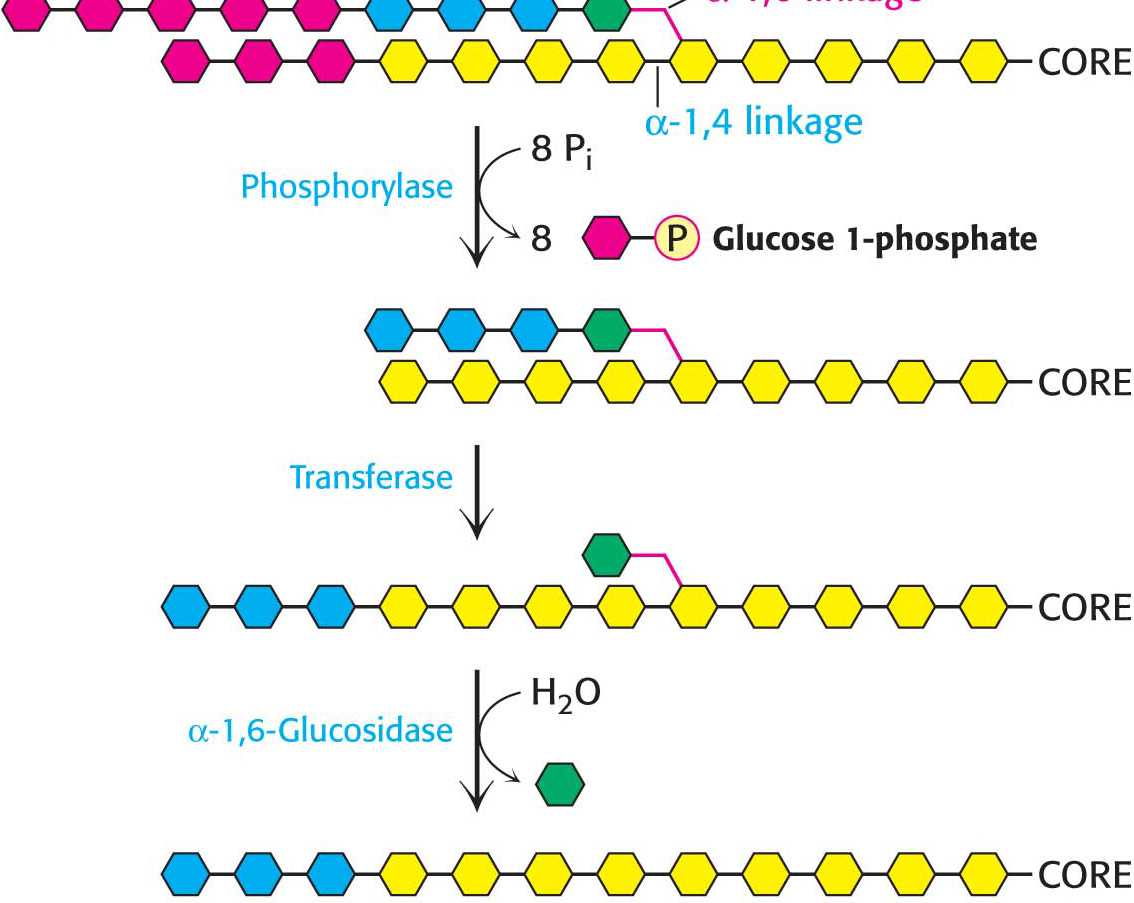
Figure 24.3: Glycogen remodeling. First, α-1,4-glycosidic bonds on each branch are cleaved by phosphorylase, leaving four residues along each branch. The transferase shifts a block of three glucosyl residues from one outer branch to the other. In this reaction, the α-1,4-glycosidic link between the blue and the green residues is broken and a new α-1,4 link between the blue and the yellow residues is formed. The green residue is then removed by α-1,6-glucosidase, leaving a linear chain with all α-1,4 linkages, suitable for further cleavage by phosphorylase.
This free glucose molecule is phosphorylated by the glycolytic enzyme hexokinase. Thus, the transferase and α-1,6-glucosidase convert the branched structure into a linear one, which paves the way for further cleavage by phosphorylase. In eukaryotes, the transferase and the α-1,6-glucosidase activities are present in a single 160-kDa polypeptide chain, providing yet another example of a bifunctional enzyme.
Phosphoglucomutase Converts Glucose 1-phosphate into Glucose 6-phosphate
Glucose 1-phosphate formed in the phosphorolytic cleavage of glycogen must be converted into glucose 6-phosphate to enter the metabolic mainstream. This shift of a phosphoryl group is catalyzed by phosphoglucomutase. Recall that this enzyme is also used in galactose metabolism. To catalyze this shift, the enzyme exchanges a phosphoryl group with the substrate (Figure 24.4). The active site of the mutase contains a phosphorylated serine residue. The phosphoryl group is transferred from the serine residue to the C-6 hydroxyl group of glucose 1-phosphate to form glucose 1,6-bisphosphate. The C-1 phosphoryl group of this intermediate is then shuttled to the same serine residue, resulting in the formation of glucose 6-phosphate and the regeneration of the phosphoenzyme.
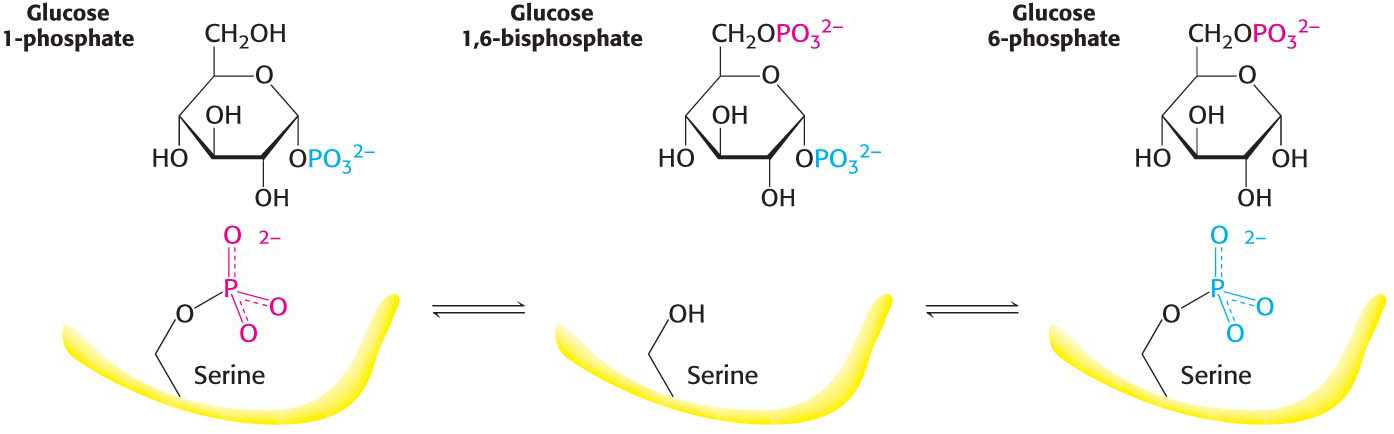
Figure 24.4: The reaction catalyzed by phosphoglucomutase. A phosphoryl group is transferred from the enzyme to the substrate, and a different phosphoryl group is transferred back to restore the enzyme to its initial state.
Liver Contains Glucose 6-phosphatase, a Hydrolytic Enzyme Absent from Muscle
A major function of the liver is to maintain a nearly constant concentration of glucose in the blood. The liver releases glucose into the blood during muscular activity and between meals. The released glucose is taken up by the brain, skeletal muscle, and red blood cells. In contrast with unmodified glucose, however, the phosphorylated glucose produced by glycogen breakdown is not transported out of cells. The liver contains a hydrolytic enzyme, glucose 6-phosphatase, that enables glucose to leave that organ. This enzyme cleaves the phosphoryl group to form free glucose and orthophosphate:
!quickquiz! QUICK QUIZ 1
What enzymes are required for the liver to release glucose into the blood when an organism is asleep and fasting?
This glucose 6-phosphatase is the same enzyme that releases free glucose at the conclusion of gluconeogenesis. It is located on the lumenal side of the smooth endoplasmic reticulum membrane. Recall that glucose 6-phosphate is transported into the endoplasmic reticulum; glucose and orthophosphate formed by hydrolysis are then shuttled back into the cytoplasm. Glucose 6-phosphatase is absent from most other tissues. These tissues retain glucose 6-phosphate for the generation of ATP. In contrast, glucose is not a major fuel for the liver.