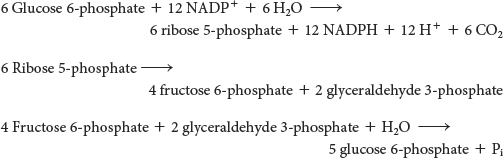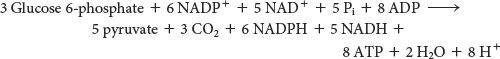The Rate of the Pentose Phosphate Pathway Is Controlled by the Concentration of NADP+
The first reaction in the oxidative branch of the pentose phosphate pathway, the dehydrogenation of glucose 6-phosphate by glucose 6-phosphate dehydrogenase, is irreversible. In fact, this reaction is rate limiting in vivo and serves as the control site for the oxidative branch of the pathway. The most important regulatory factor is the concentration of NADP+, the electron acceptor in the oxidation steps of the pentose phosphate pathway. When there is an excess of NADPH, there is a small amount of NADP+ in cells. At low concentrations of NADP+, the activity of the three enzymes participating in the oxidation step is low because there are fewer oxidizing agents to move the reaction forward. The inhibitory effect of low concentrations of NADP+ is enhanced by the fact that NADPH, which is present in 70-fold greater concentration than is NADP+ in liver cytoplasm, competes with NADP+ in binding to the enzyme, thereby preventing the oxidation of glucose 6-phosphate. The effect of the NADP+ concentration on the rate of the oxidative phase ensures that the generation of NADPH is tightly coupled to its use in reductive biosyntheses or protection against oxidative stress. The nonoxidative phase of the pentose phosphate pathway is controlled primarily by the availability of substrates.
The Fate of Glucose 6-phosphate Depends on the Need for NADPH, Ribose 5-phosphate, and ATP
We can grasp the “Swiss Army knife-like” versatility of the pentose phosphate pathway, as well as its interplay with glycolysis and gluconeogenesis, by examining the metabolism of glucose 6-phosphate in four different metabolic situations (Figure 26.3).
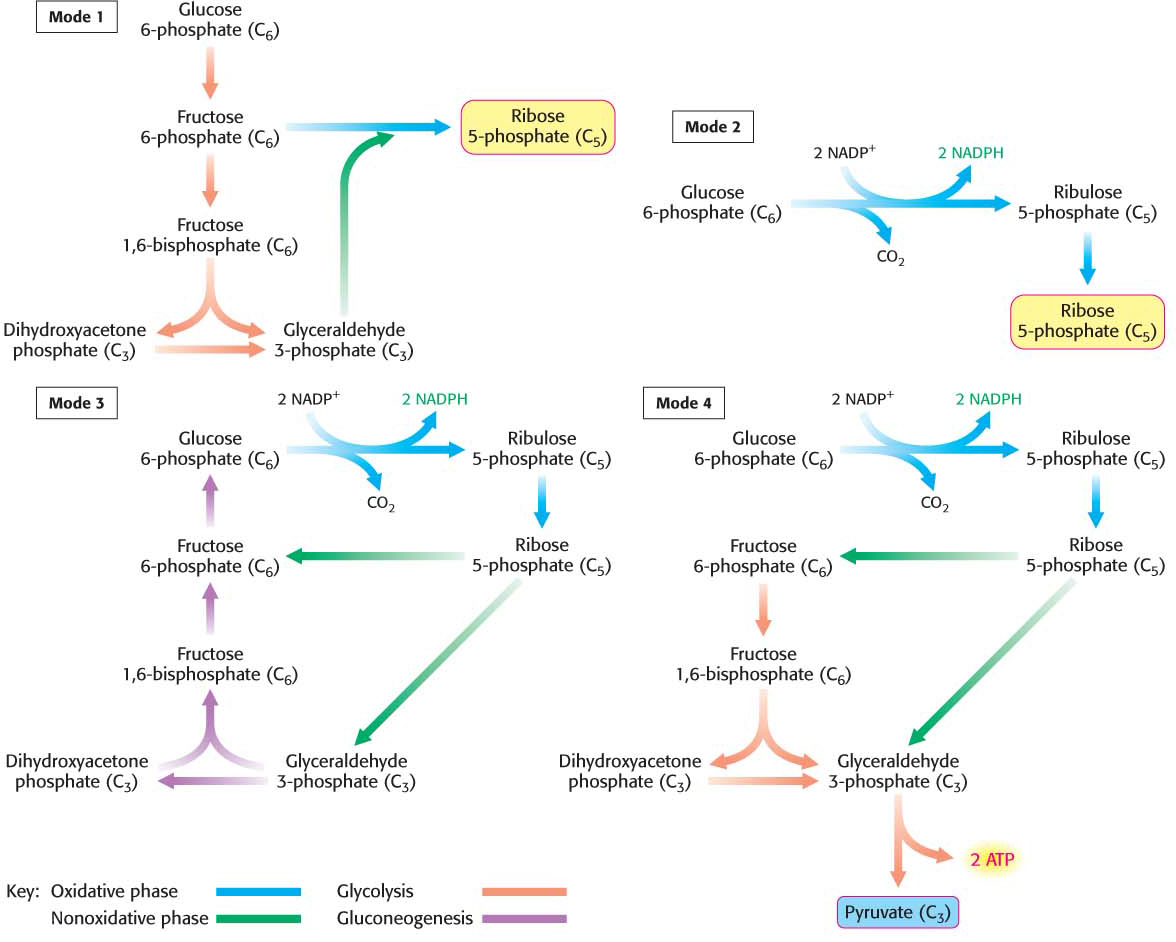
Figure 26.3: Four modes of the pentose phosphate pathway. Major products are shown in color.
Mode 1. The need for ribose 5-phosphate is greater than the need for NADPH. For example, rapidly dividing cells need ribose 5-phosphate for the synthesis of nucleotide precursors of DNA. Most of the glucose 6-phosphate is converted into fructose 6-phosphate and glyceraldehyde 3-phosphate by the glycolytic pathway. The nonoxidative phase of the pentose phosphate pathway then converts two molecules of fructose 6-phosphate and one molecule of glyceraldehyde 3-phosphate into three molecules of ribose 5-phosphate by a reversal of the reactions described earlier. The stoichiometry of mode 1 is
Mode 2. The needs for NADPH and ribose 5-phosphate are balanced. Under these conditions, glucose 6-phosphate is processed to one molecule of ribulose 5-phosphate while generating two molecules of NADPH. Ribulose 5-phosphate is then converted into ribose 5-phosphate. The stoichiometry of mode 2 is

Mode 3. The need for NADPH is greater than the need for ribose 5-phosphate. For example, the liver requires a large amount of NADPH for the synthesis of fatty acids (Table 26.3). In this case, glucose 6-phosphate is completely oxidized to CO2. Three groups of reactions are active in this situation. First, the oxidative phase of the pentose phosphate pathway forms two molecules of NADPH and one molecule of ribose 5-phosphate. Then, ribose 5-phosphate is converted into fructose 6-phosphate and glyceraldehyde 3-phosphate by the nonoxidative phase. Finally, glucose 6-phosphate is resynthesized from fructose 6-phosphate and glyceraldehyde 3-phosphate by the gluconeogenic pathway. In essence, ribose 5-phosphate produced by the pentose phosphate pathway is recycled into glucose 6-phosphate by the nonoxidative phase of the pathway and by some of the enzymes of the gluconeogenic pathway. The stoichiometries of these three sets of reactions are
The sum of the mode 3 reactions is
Thus, the equivalent of glucose 6-phosphate can be completely oxidized to CO2 with the concomitant generation of NADPH.

Table 26.3 Tissues with active pentose phosphate pathways
Mode 4. Both NADPH and ATP are required. Alternatively, ribulose 5-phosphate formed from glucose 6-phosphate can be converted into pyruvate when reducing power and ATP are needed. Fructose 6-phosphate and glyceraldehyde 3-phosphate derived from the nonoxidative reactions of the pentose phosphate pathway enter the glycolytic pathway rather than reverting to glucose 6-phosphate. In this mode, ATP and NADPH are concomitantly generated, and five of the six carbon atoms of glucose 6-phosphate emerge in pyruvate. The stoichiometry of mode 4 is
Pyruvate formed by these reactions can be oxidized to generate more ATP or it can be used as a building block in a variety of biosyntheses.
!clinic! CLINICAL INSIGHT: The Pentose Phosphate Pathway Is Required For Rapid Cell Growth
Rapidly dividing cells, such as cancer cells, require ribose 5-phosphate for nucleic acid synthesis and NADPH for reductive biosynthesis. Recall that rapidly dividing cells switch to aerobic glycolysis to meet their ATP needs. Glucose 6-phosphate and glycolytic intermediates are then used to generate NADPH and ribose 5-phosphate using the pentose phosphate pathway as described in modes 1 and 3 above. The diversion of glycolytic intermediates into the nonoxidative phase is facilitated by the expression of the gene for a pyruvate kinase isozyme PKM. Recall that pyruvate kinase catalyzes the conversion of phosphoenolpyruvate into pyruvate with the synthesis of a molecule of ATP. PKM has a low catalytic activity creating a bottleneck in the glycolytic pathway. Glycolytic intermediates accumulate and are then used by the pentose phosphate pathway to synthesize ribose 5-phosphate. The shunting of phosphorylated intermediates into the nonoxidative phase of the pentose phosphate pathway is further enabled by the inhibition of triose phosphate isomerase by phosphoenolpyruvate, the substrate of PKM.