26.3 Glucose 6-phosphate Dehydrogenase Lessens Oxidative Stress
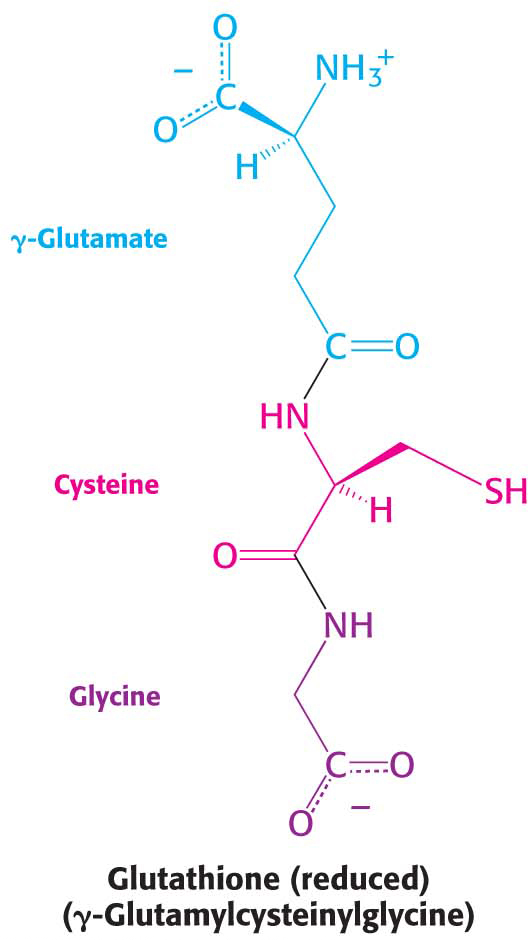
The NADPH generated by the pentose phosphate pathway plays a vital role in protecting the cells from reactive oxygen species (ROS). Reactive oxygen species generated in oxidative metabolism inflict damage on all classes of macromolecules and can ultimately lead to cell death. Indeed, ROS are implicated in a number of human diseases. Reduced glutathione (GSH), a tripeptide with a free sulfhydryl group, combats oxidative stress by reducing ROS to harmless forms and in the process is oxidized to GSSG, two molecules of glutathione joined by a disulfide bond. GSSG must be reduced to regenerate GSH. The reducing power is supplied by the NADPH generated by glucose 6-
!clinic! CLINICAL INSIGHT: Glucose 6-phosphate Dehydrogenase Deficiency Causes a Drug-Induced Hemolytic Anemia
The importance of the pentose phosphate pathway is highlighted by the anomalous response of people to certain drugs. For instance, pamaquine, the first synthetic antimalarial drug introduced in 1926, was associated with the appearance of severe and mysterious ailments. Most patients tolerated the drug well, but a few developed severe symptoms within a few days after therapy was started. Their urine turned black, jaundice developed, and the hemoglobin content of the blood dropped sharply. In some cases, massive destruction of red blood cells caused death.
This drug-
482
Pamaquine sensitivity is not simply a historical oddity about malaria treatment many decades ago. Primaquine, an antimalarial closely related to pamaquine, is widely used in malaria-
How can we explain hemolysis caused by pamaquine, primaquine, and vicine biochemically? These chemicals are oxidative agents that generate peroxides, reactive oxygen species that can damage membranes as well as other biomolecules. Peroxides are normally eliminated by the enzyme glutathione peroxidase, which uses reduced glutathione as a reducing agent:

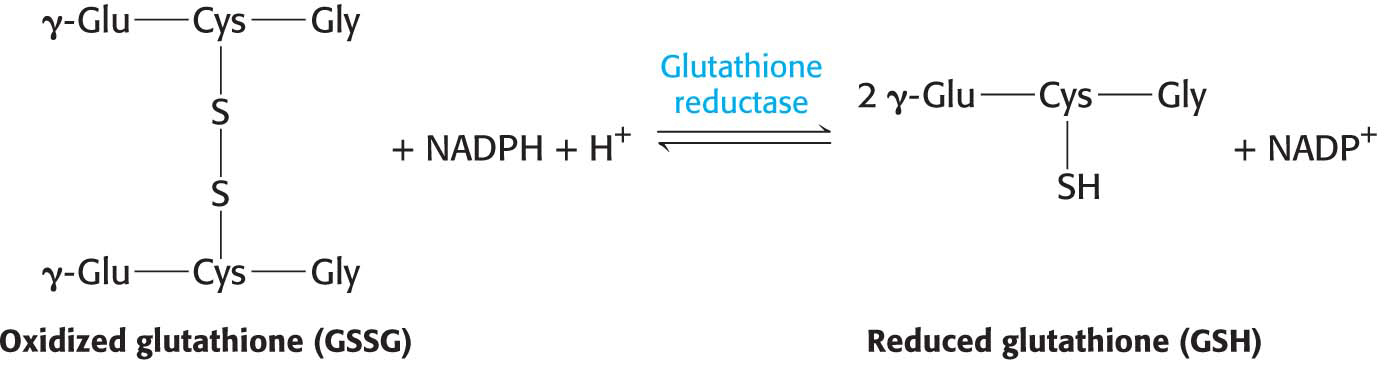
The major role of NADPH in red cells is to reduce the disulfide form of glutathione to the sulfhydryl form. The enzyme that catalyzes the regeneration of reduced glutathione is glutathione reductase:
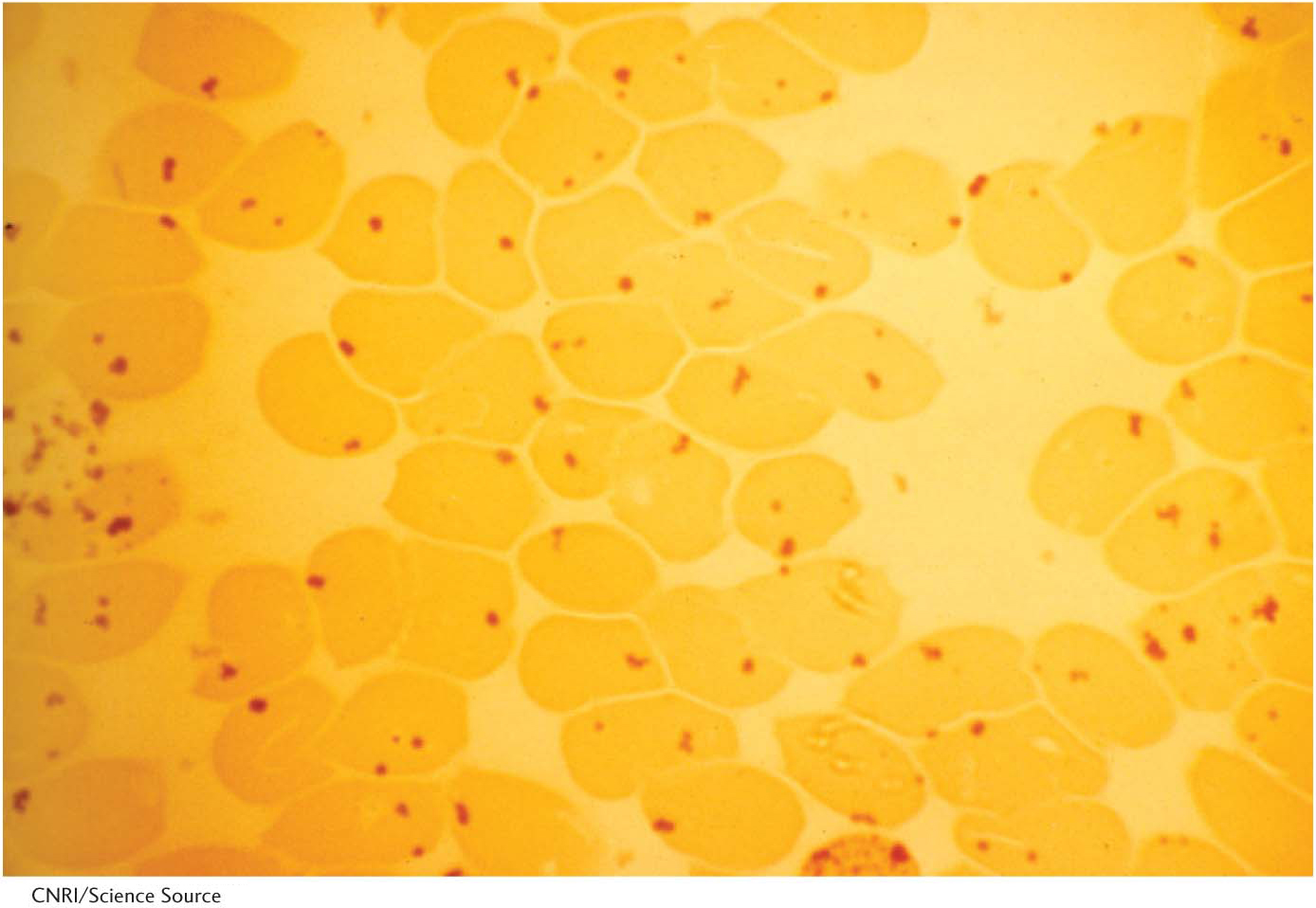
Red blood cells with a low concentration of reduced glutathione are more susceptible to hemolysis. In the absence of glucose 6-
Reduced glutathione is also essential for maintaining the normal structure of red blood cells by preserving the structure of hemoglobin. The reduced form of glutathione serves as a sulfhydryl buffer that keeps the residues of hemoglobinin the reduced sulfhydryl form. Without adequate amounts of reduced glutathione, the hemoglobin sulfhydryl groups can no longer be maintained in the reduced form. Hemoglobin molecules then cross-
483
!bio! BIOLOGICAL INSIGHT: A Deficiency of Glucose 6-phosphate Dehydrogenase Confers an Evolutionary Advantage in Some Circumstances
The incidence of the most common form of glucose 6-
The ability of glucose 6-