29.1 Phosphatidate Is a Precursor of Storage Lipids and Many Membrane Lipids
✓ 5 Describe the relation between triacylglycerol synthesis and phospholipid synthesis.
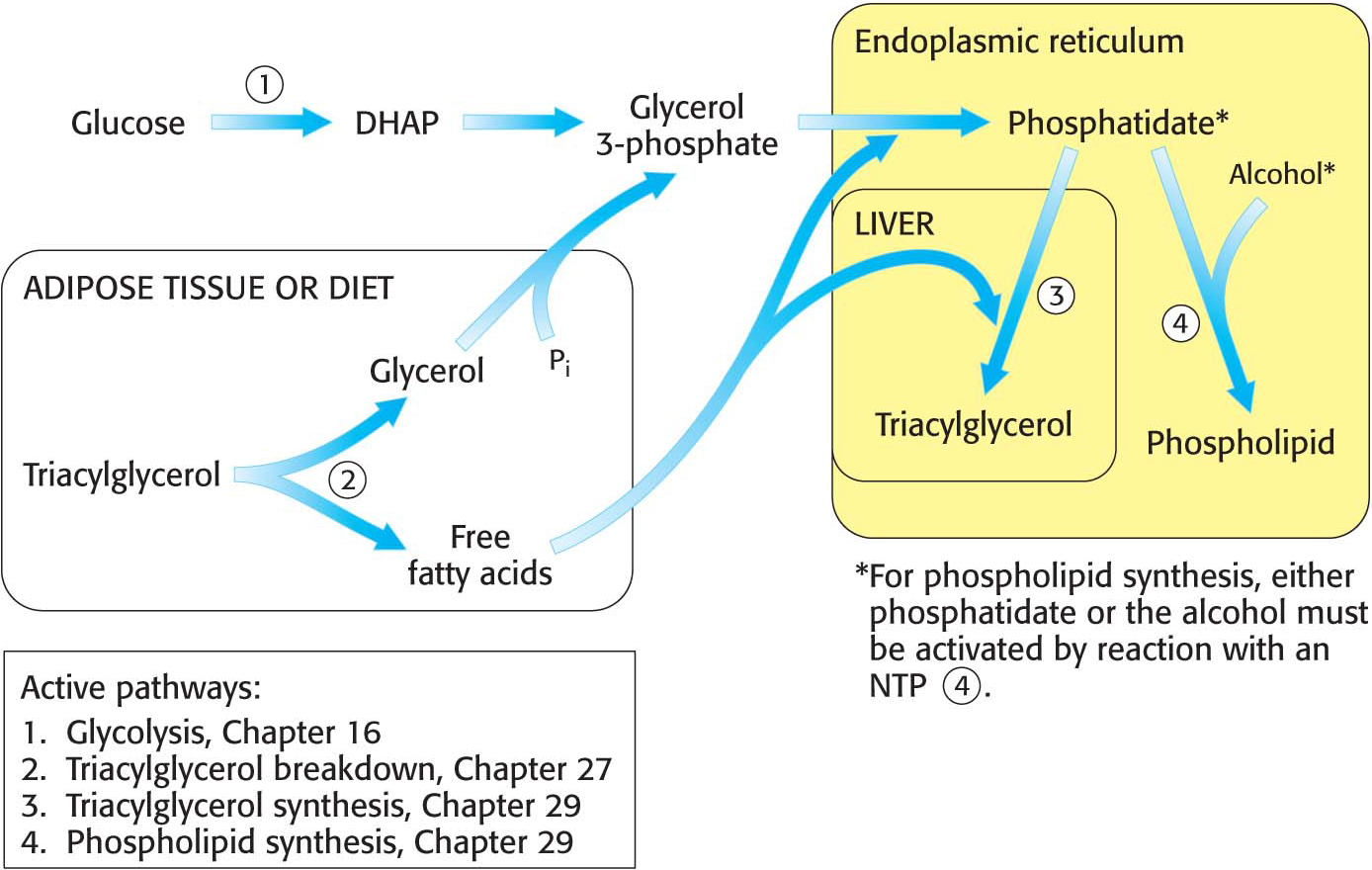
Figure 29.1 provides a broad view of lipid synthesis. Both triacylglycerol synthesis and phospholipid synthesis begin with the precursor phosphatidate (diacylglycerol 3-
524
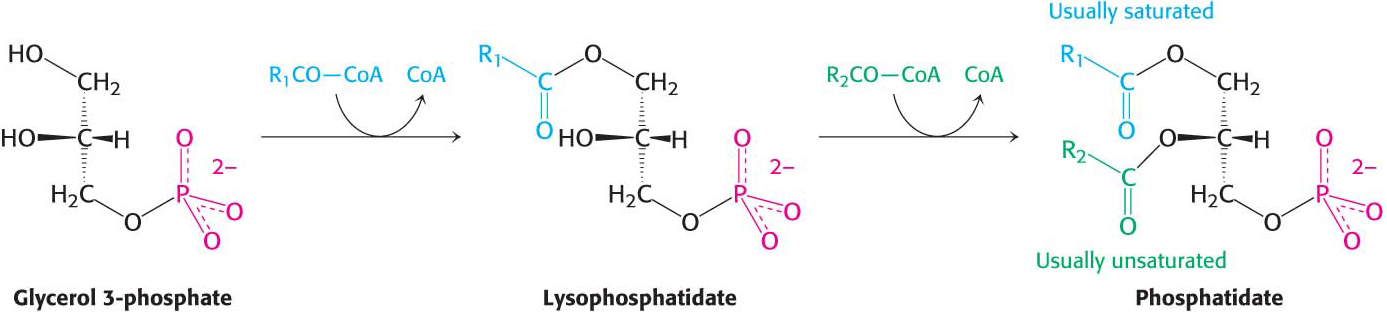
Triacylglycerol Is Synthesized from Phosphatidate in Two Steps
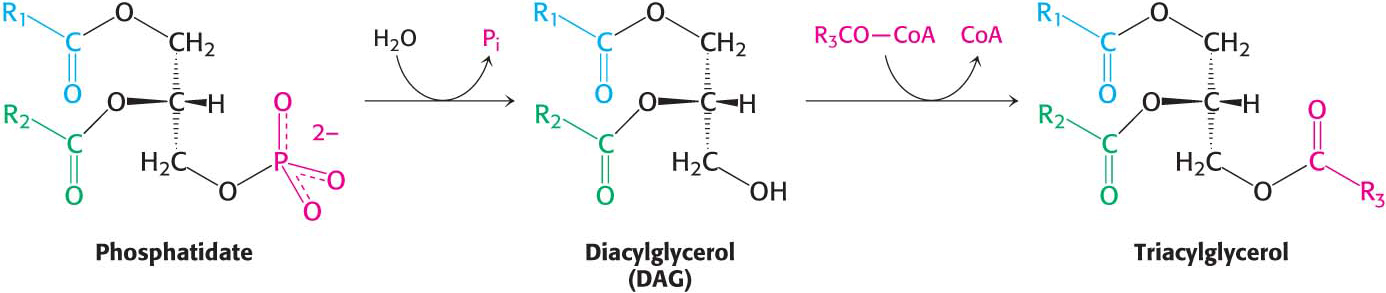
The pathways diverge at phosphatidate. The synthesis of triacylglycerol is completed by a triacylglycerol synthetase complex that is bound to the endoplasmic reticulum membrane. Phosphatidate is hydrolyzed to give diacylglycerol (DAG), which is then acylated to a triacylglycerol:
The liver is the primary site of triacylglycerol synthesis. From the liver, triacylglycerols are transported to muscles for use as a fuel or to adipose tissue for storage. Approximately 85% of a nonobese person’s energy is stored as triacylglycerols, mainly in adipose tissue.
Phospholipid Synthesis Requires Activated Precursors
Phosphatidate is also a precursor for phospholipids. Phospholipid synthesis, which takes place in the endoplasmic reticulum, requires the combination of a diacylglycerol with an alcohol. As in most anabolic reactions, one of the components must be activated. In this case, either of the two components may be activated, depending on the source of the reactants.
525
Synthesis from an activated diacylglycerolThis pathway starts with the reaction of phosphatidate with cytidine triphosphate (CTP) to form cytidine diphosphodiacylglycerol (CDP-
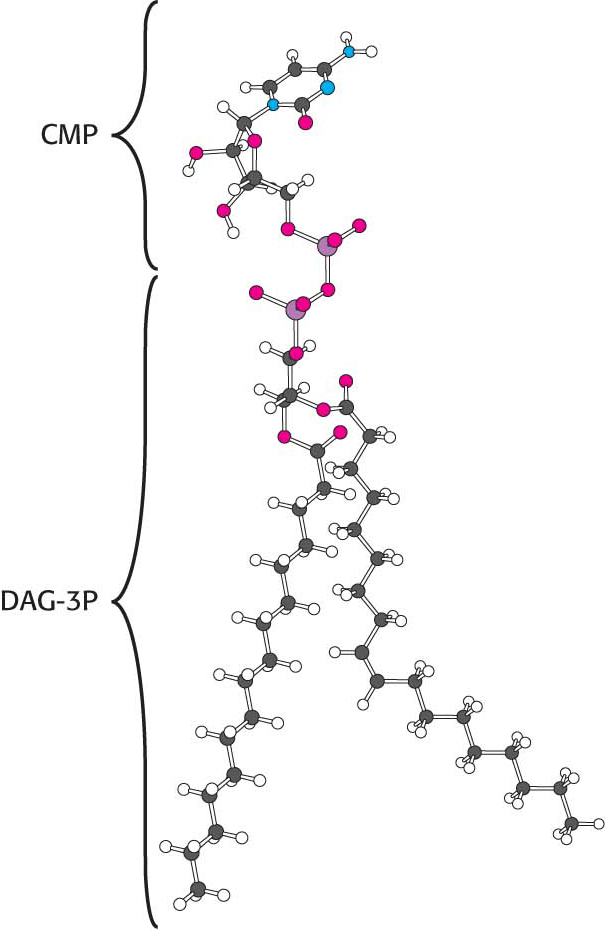
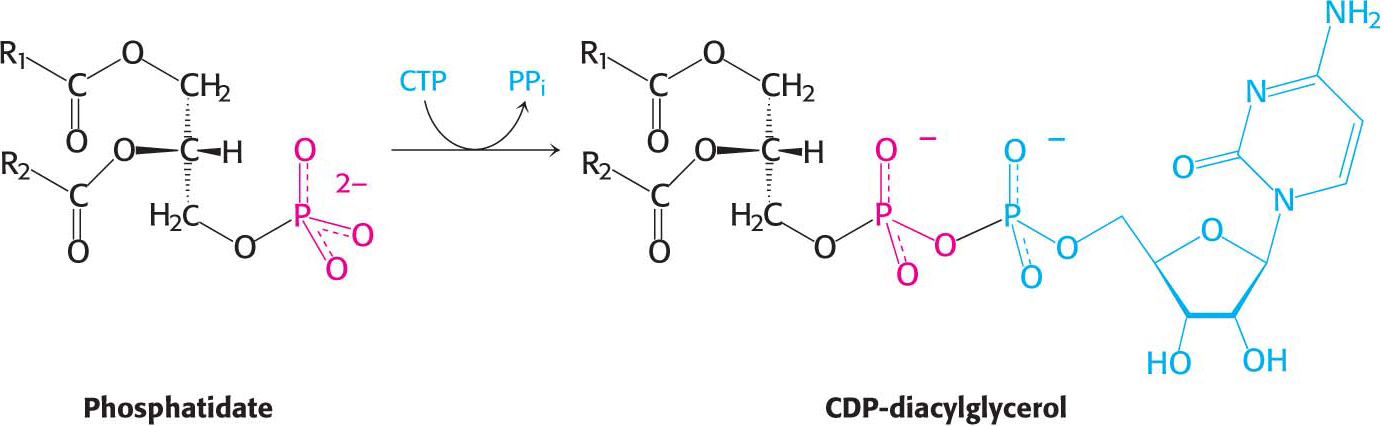
The activated phosphatidyl unit then reacts with the hydroxyl group of an alcohol. If the alcohol is inositol, the products are phosphatidylinositol and cytidine monophosphate (CMP). Subsequent phosphorylations of phosphatidylinositol catalyzed by specific kinases lead to the synthesis of phosphatidylinositol 4,5-
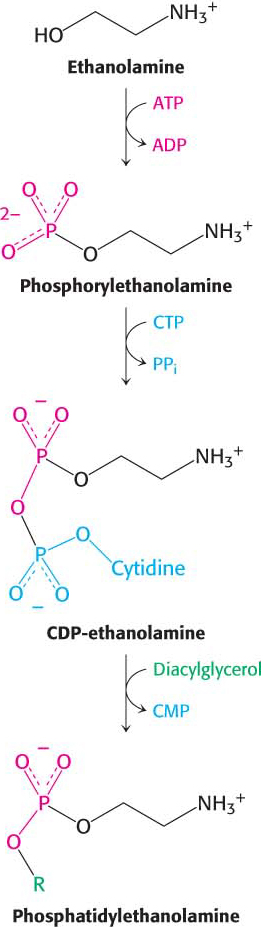
Synthesis from an activated alcoholPhosphatidylethanolamine in mammals can be synthesized from the alcohol ethanolamine through the formation of CDP-
526
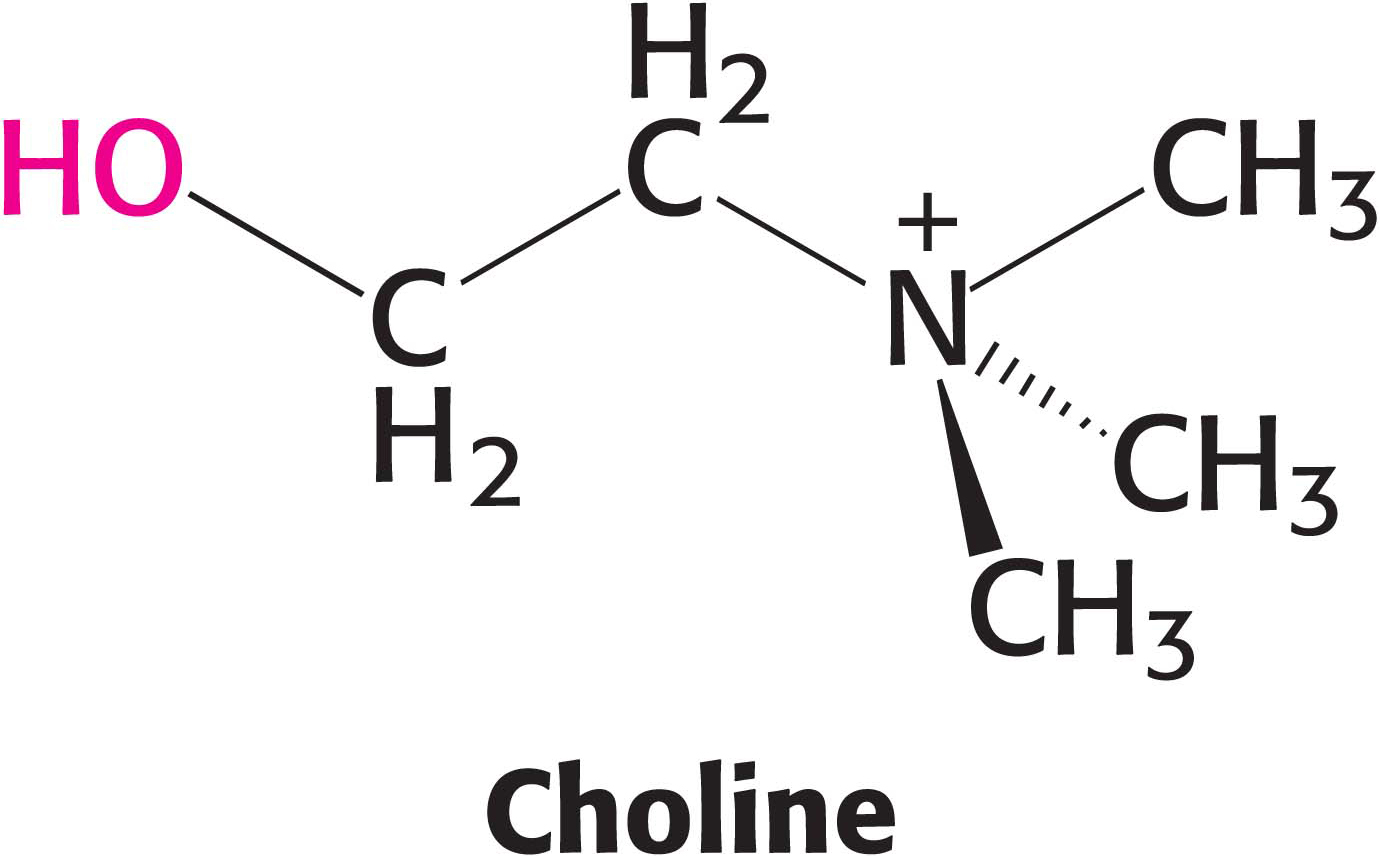
!clinic! CLINICAL INSIGHT: Phosphatidylcholine Is an Abundant Phospholipid
The most common phospholipid in mammals is phosphatidylcholine, comprising approximately 50% of the membrane mass. Dietary choline is activated in a series of reactions analogous to those in the activation of ethanolamine. CTP-
The importance of phosphatidylcholine is attested to by the fact that the liver possesses an enzyme, phosphatidylethanolamine methyltransferase, that synthesizes phosphatidylcholine from phosphatidylethanolamine when dietary choline is insufficient. The amino group of this phosphatidylethanolamine is methylated three times to form phosphatidylcholine. S-Adenosylmethionine is the methyl donor (Chapter 31):
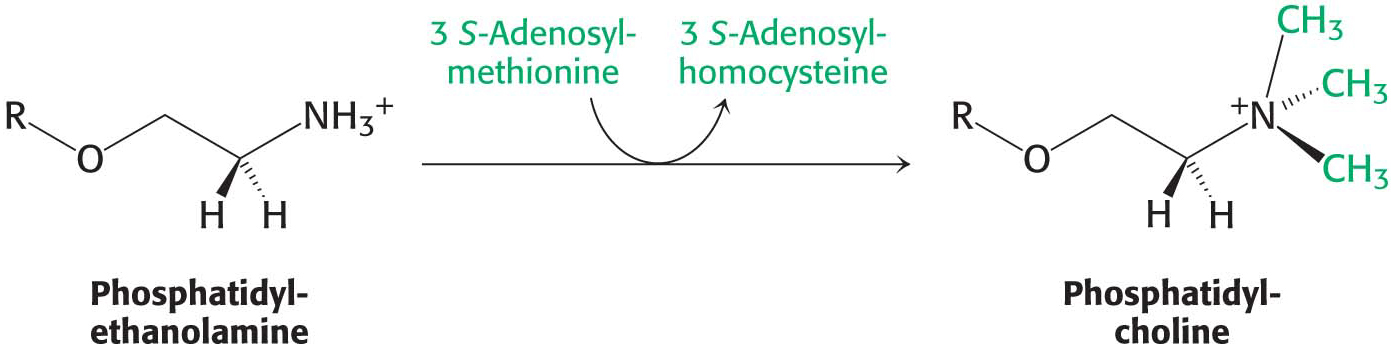
Thus, phosphatidylcholine can be produced by two distinct pathways in mammals, ensuring that this phospholipid can be synthesized even if the components for one pathway are in limited supply.
Sphingolipids Are Synthesized from Ceramide
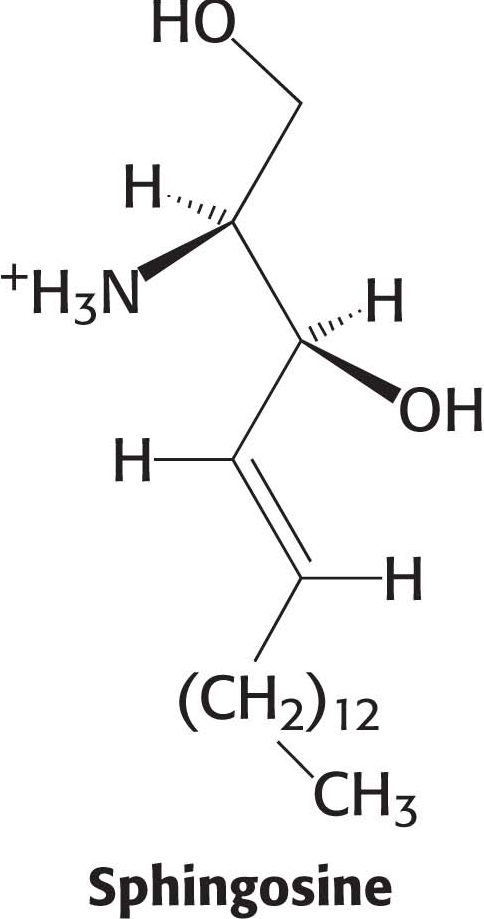
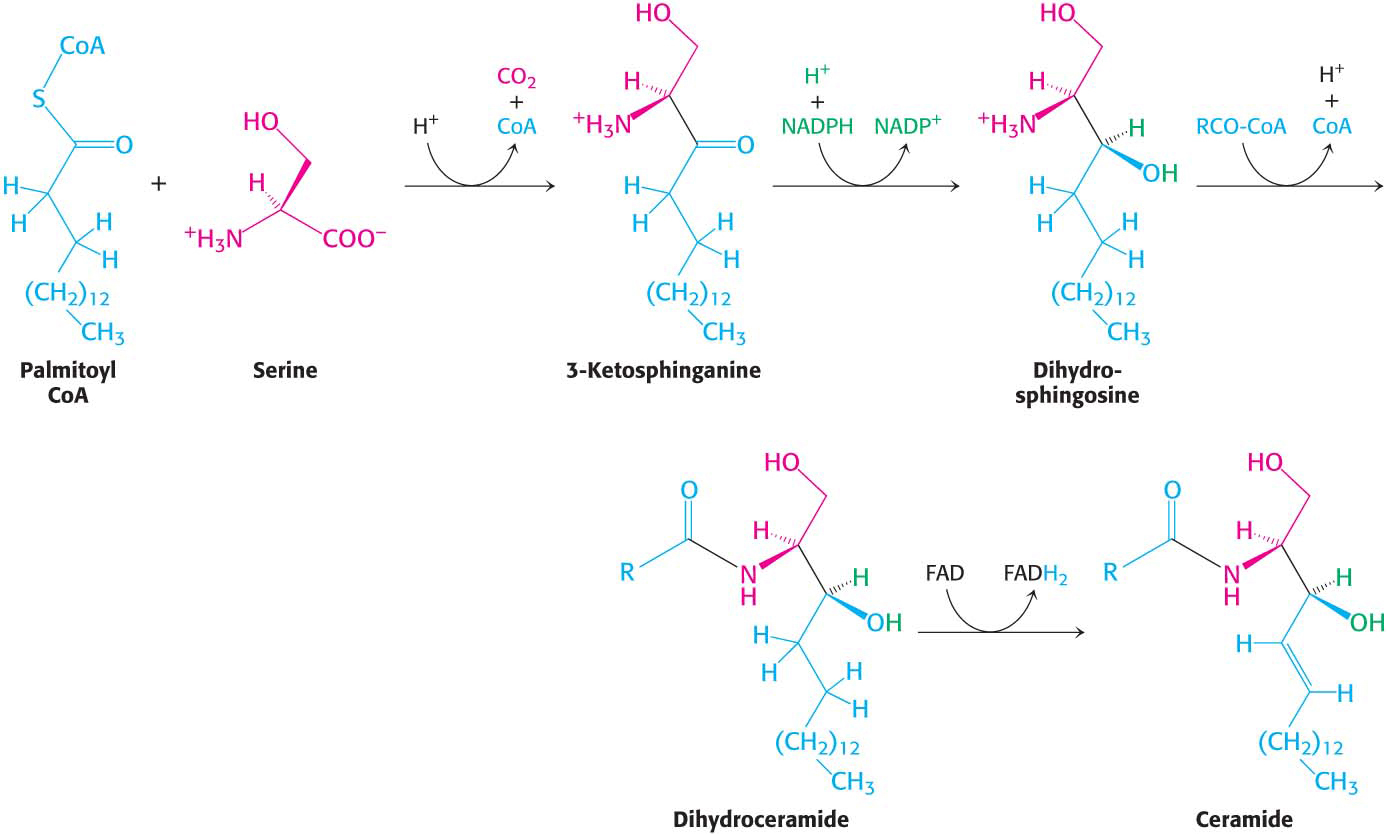
Phospholipids, with their glycerol backbones, are not the only type of membrane lipid. Sphingolipids, with a backbone of sphingosine rather than glycerol, are found in the plasma membranes of all eukaryotic cells, although the concentration is highest in the cells of the central nervous system. To synthesize a sphingolipid, palmitoyl CoA and serine condense to form 3-
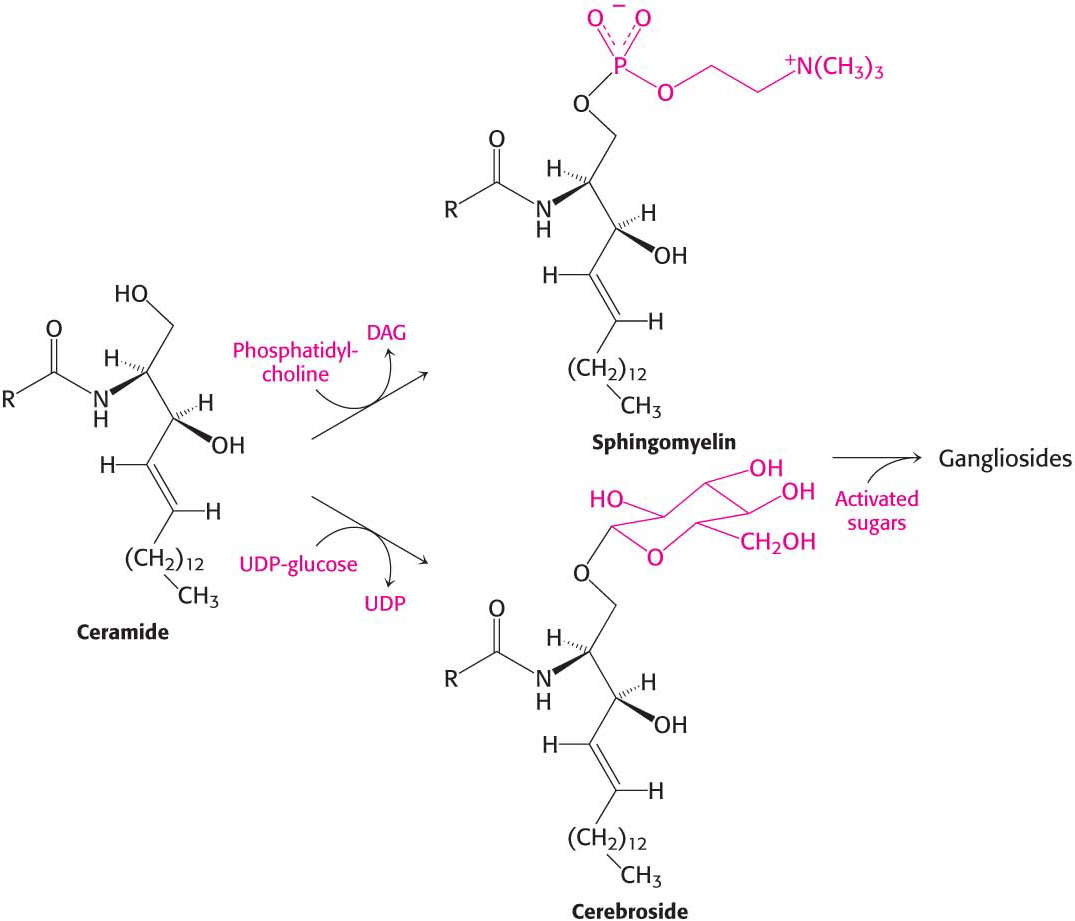
The terminal hydroxyl group of ceramide is also substituted to form a variety of sphingolipids (Figure 29.4):
In sphingomyelin, a component of the myelin sheath covering many nerve fibers, the substituent is phosphorylcholine.
In a cerebroside, also a component of myelin, the substituent is glucose or galactose.
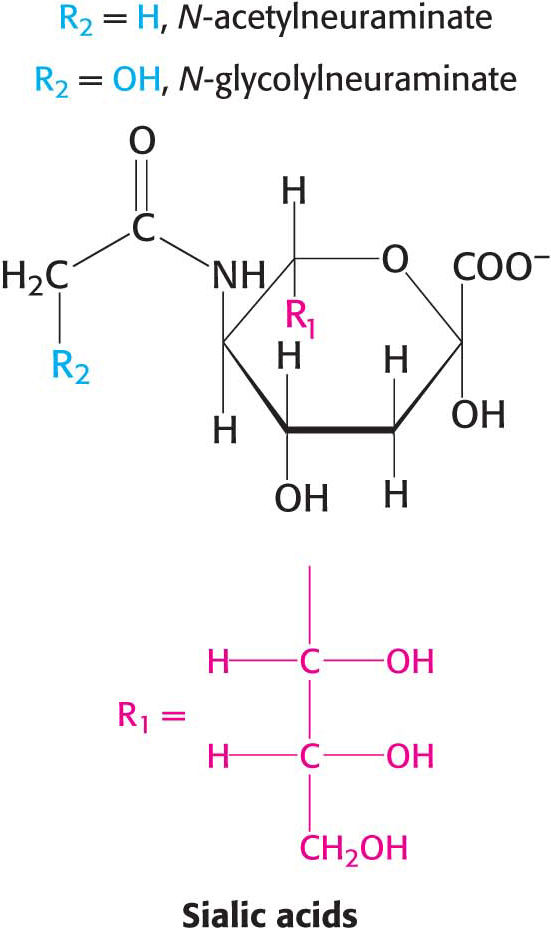
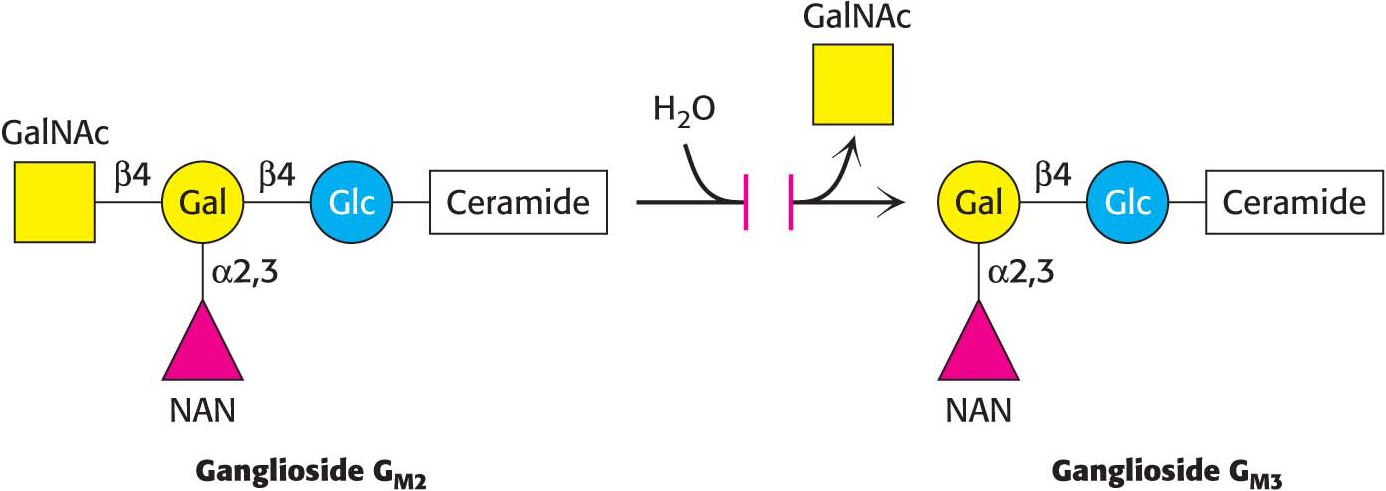
In a ganglioside, an oligosaccharide containing at least one sialic acid is linked to the terminal hydroxyl group of ceramide by a glucose residue.
527
!quickquiz! QUICK QUIZ 1
Describe the roles of glycerol 3-
!clinic! CLINICAL INSIGHT: Gangliosides Serve as Binding Sites for Pathogens
Ganglioside-
528
!clinic! CLINICAL INSIGHT: Disrupted Lipid Metabolism Results in Respiratory Distress Syndrome and Tay–Sachs Disease
Disruptions in lipid metabolism are responsible for a host of diseases. We will briefly examine two such conditions. Respiratory distress syndrome is a pathological condition resulting from a failure in the biosynthesis of dipalmitoylphosphatidylcholine. This phospholipid, in conjunction with specific proteins and other phospholipids, is found in the extracellular fluid that surrounds the alveoli of the lung, where it decreases the surface tension of the fluid to prevent lung collapse at the end of the expiration phase of breathing. Premature infants may suffer from respiratory distress syndrome because their immature lungs do not synthesize enough dipalmitoylphosphatidylcholine.
Whereas respiratory distress syndrome results from failure in biosynthesis, Tay–
As a consequence, neurons become significantly swollen with lipid-
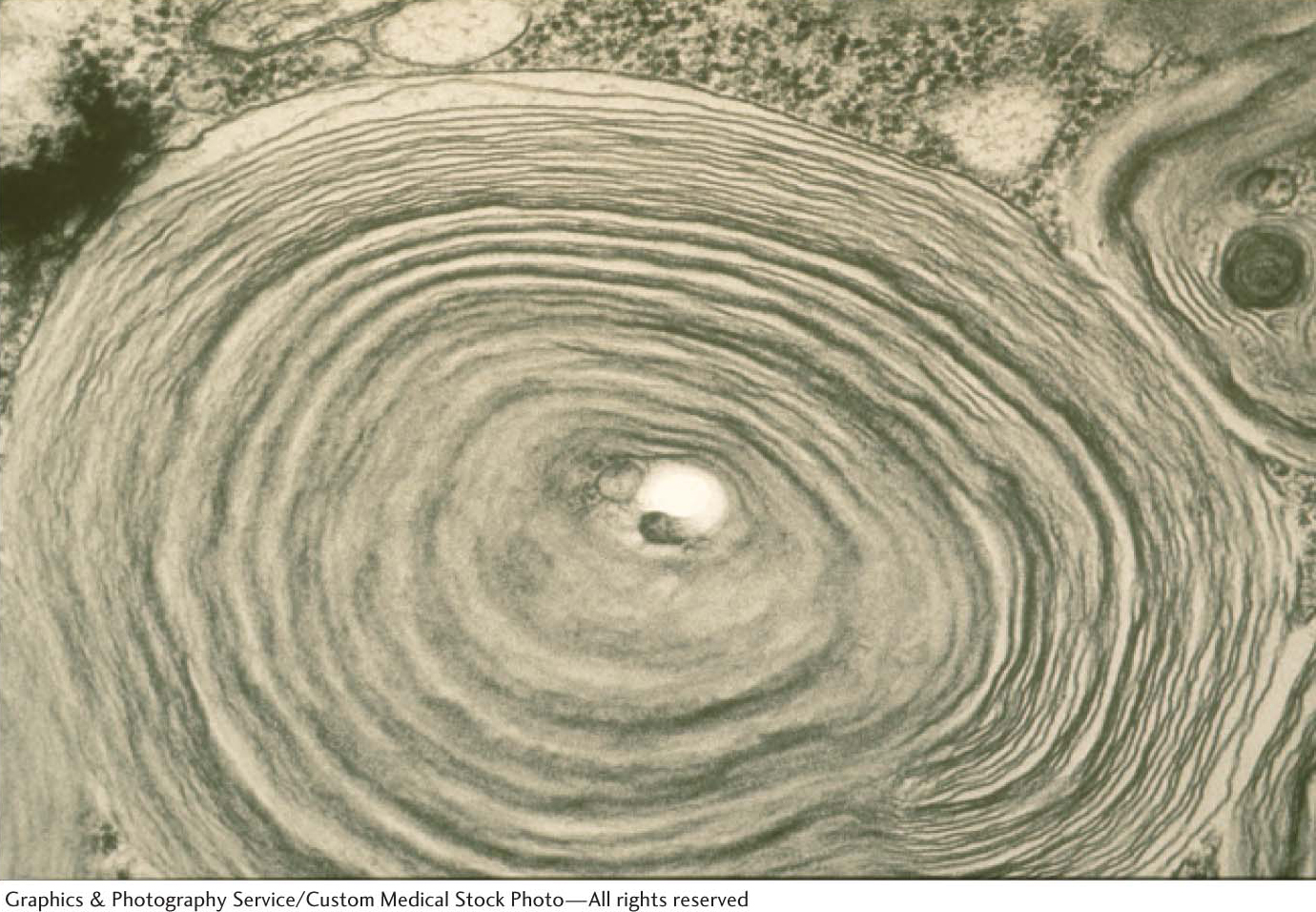
529
Tay–
Phosphatidic Acid Phosphatase Is a Key Regulatory Enzyme in Lipid Metabolism
Although the details of the regulation of lipid synthesis remain to be elucidated, evidence suggests that phosphatidic acid phosphatase (PAP), working in concert with diacylglycerol kinase, plays a key role in lipid synthesis regulation. Phosphatidic acid phosphatase, also called lipin 1 in mammals, controls the extent to which triacylglycerols are synthesized relative to phospholipids and regulates the type of phospholipid synthesized (Figure 29.7). For instance, when PAP activity is high, phosphatidate is dephosphorylated and diacylglycerol is produced, which can react with the appropriate activated alcohols to yield phosphatidylethanolamine, phosphatidylserine, or phosphatidylcholine. Diacylglycerols can also be converted into triacylglycerols, and evidence suggests that the formation of triacylglycerols may act as a fatty acid buffer, which helps to regulate the levels of diacylglycerol and sphingolipids, both of which serve signaling functions.
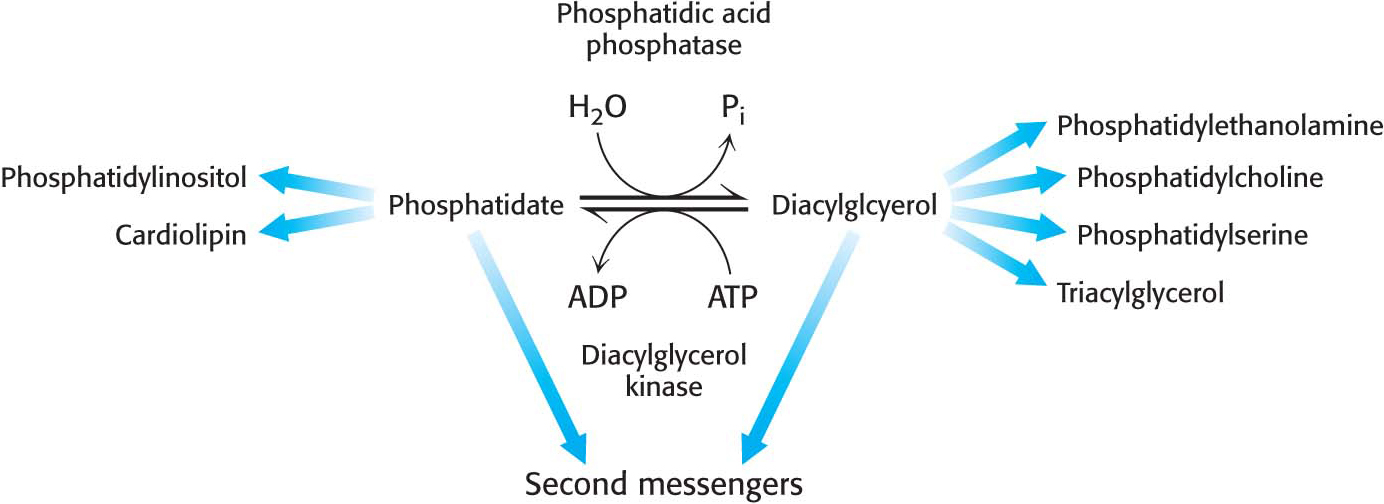
When PAP activity is lower, phosphatidate is used as a precursor for different phospholipids, such as phosphatidylinositol and cardiolipin. Moreover, phosphatidate is a signal molecule itself. Phosphatidate regulates the growth of endoplasmic reticulum and nuclear membranes and acts as a cofactor that stimulates the expression of genes in phospholipid synthesis.
What are the signal molecules that regulate the activity of PAP? CDP-
Studies in mice clearly show the importance of PAP for the regulation of fatty acid synthesis. The loss of PAP function prevents normal adipose-