2.3 Weak Interactions Are Important Biochemical Properties
✓ 5 Describe the types of noncovalent, reversible interactions and explain why reversible interactions are important in biochemistry.
Readily reversible, noncovalent molecular interactions are essential interactions in the flow of energy and information. Such weak, noncovalent forces play roles in the faithful replication of DNA, the folding of proteins into elaborate three-
Electrostatic Interactions Are Between Electrical Charges
Electrostatic interactions, also called ionic bonds or salt bridges, are the interactions between distinct electrical charges on atoms. They usually take place between atoms bearing a completely negative charge and a completely positive charge. The energy of an electrostatic interaction between two ions is given by Coulomb’s law:
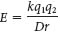
where E is the force, q1 and q2 are the charges on the two atoms (in units of the electronic charge), r is the distance between the two atoms (in angstroms), D is the dielectric constant (which accounts for the effects of the intervening medium), and k is the proportionality constant. Thus, the electrostatic interaction between two atoms bearing single opposite charges varies inversely with the square of the distance separating them as well as with the nature of the intervening medium. Electrostatic interactions are strongest in a vacuum, where D = 1. The distance for maximal bond strength is about 3 Å. Because of its polar characteristics, water (which has a dielectric constant of 80) weakens electrostatic interactions. Conversely, electrostatic interactions are maximized in an uncharged environment. For instance, the electrostatic interaction between two ions bearing single opposite charges separated by 3 Å in water has an energy of −5.8 kJ mol−1 (−1.4 kcal mol−1), whereas that between the same two ions separated by 3 Å in a nonpolar solvent such as hexane (which has a dielectric constant of 2) has an energy of −231 kJ mol−1 (−55 kcal mol−1). (Note: One kilojoule, abbreviated kJ, is equivalent to 0.239 kilocalorie, abbreviated kcal.)
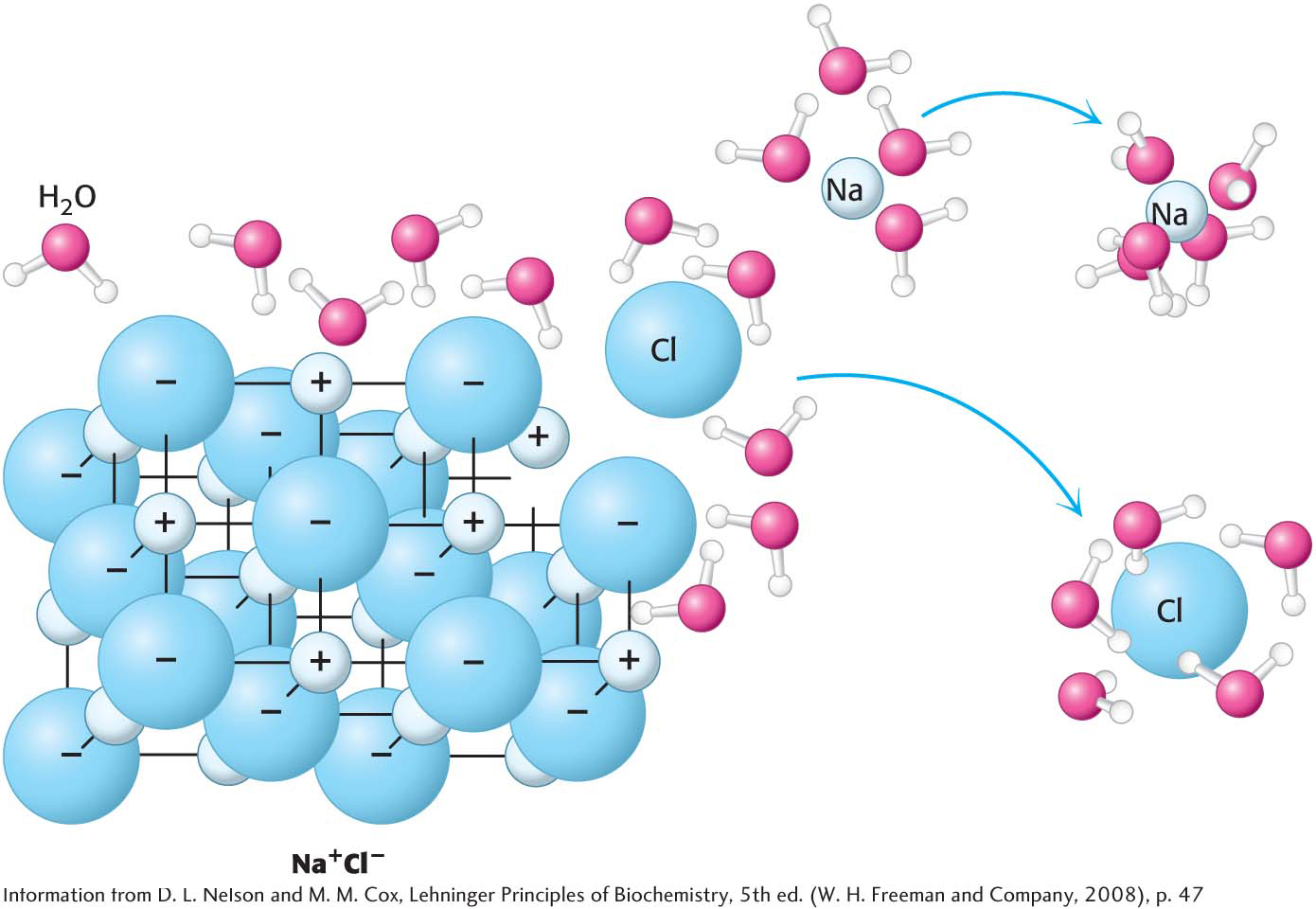
Why does water weaken electrostatic interactions? Consider what happens when a grain of salt, NaCl, is added to water. Even in its crystalline form, salt is more appropriately represented in the ionic form, Na+Cl−. The salt dissolves—
21
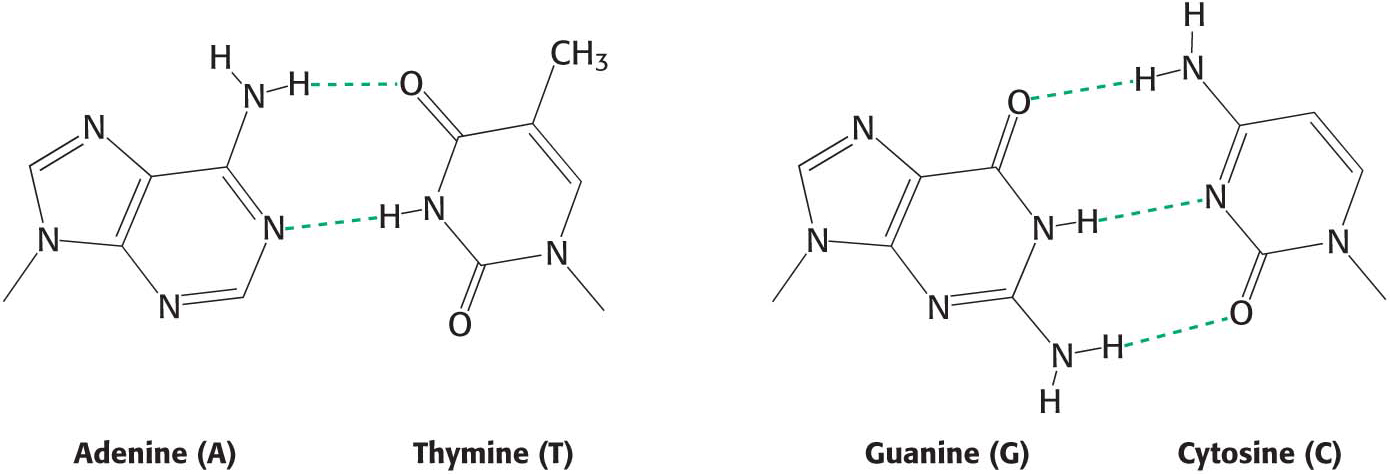
Hydrogen Bonds Form Between an Electronegative Atom and Hydrogen
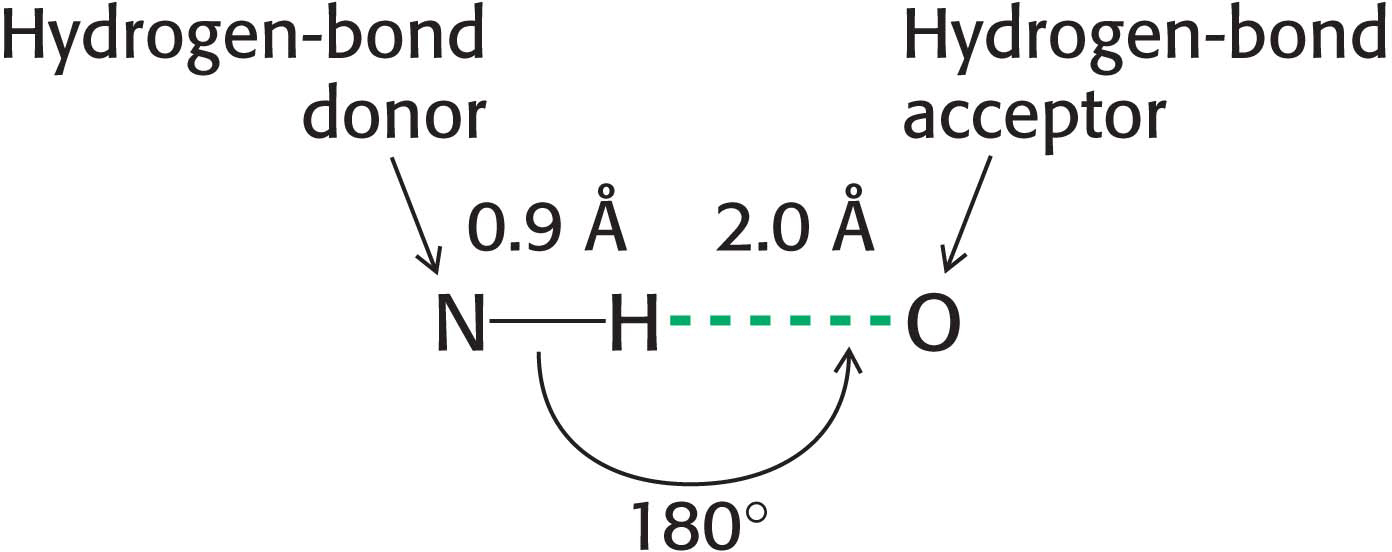
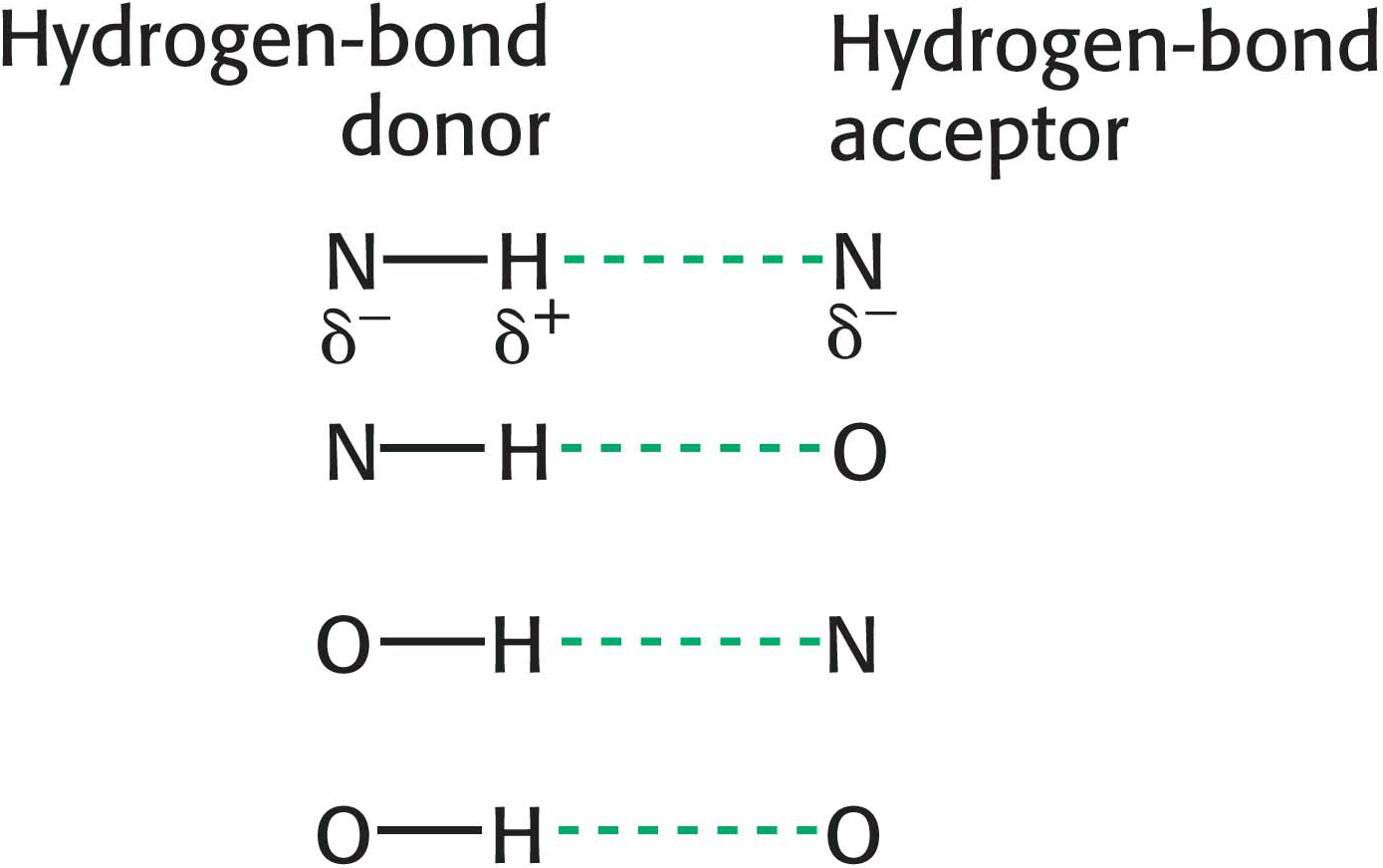
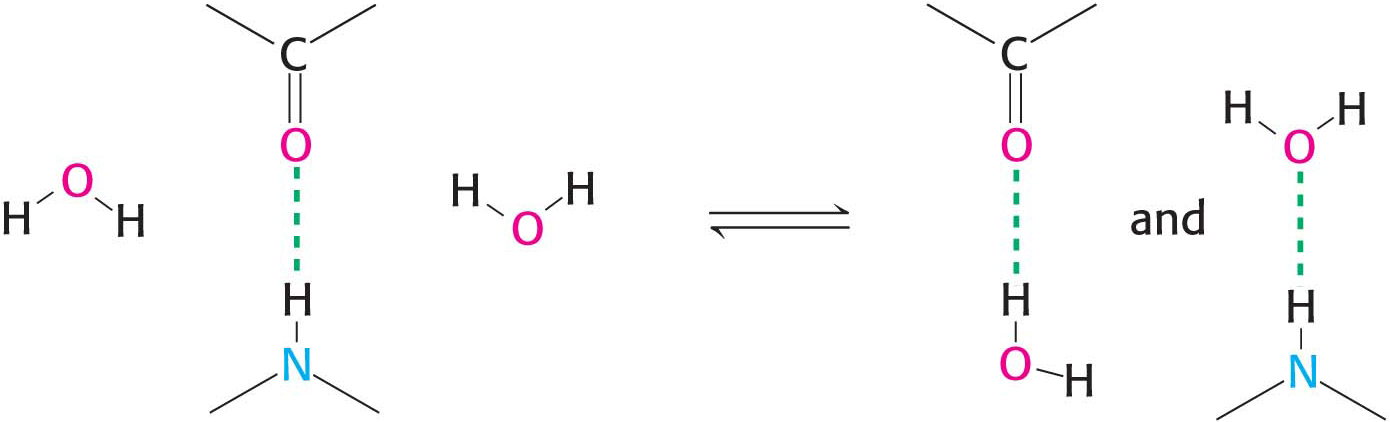
Hydrogen bonds are not unique to water molecules; the unequal distribution of charges that permit hydrogen-
van der Waals Interactions Depend on Transient Asymmetry in Electrical Charge
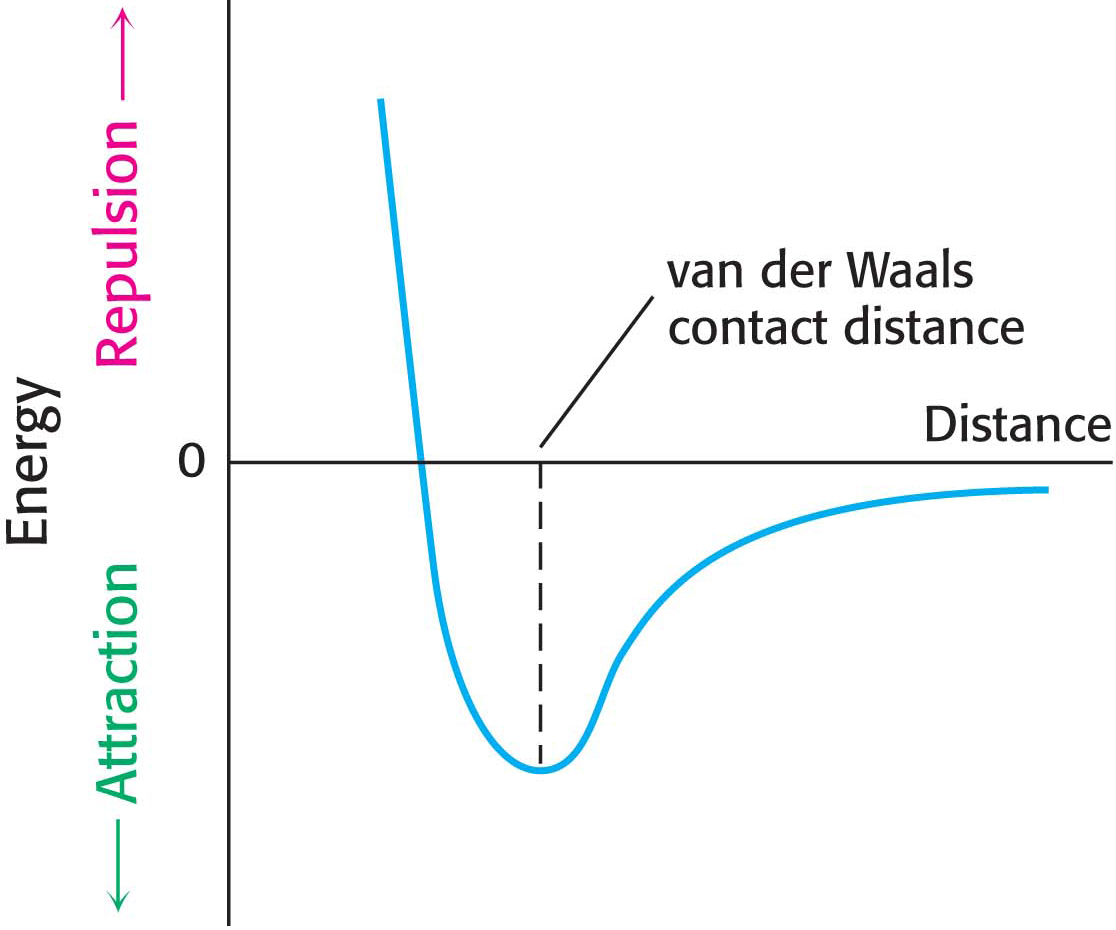
Many important biomolecules are neither polar nor charged. Nonetheless, such molecules can interact with each other electrostatically by a van der Waals interaction. The basis of a van der Waals interaction is that the distribution of electronic charge around an atom changes with time, and, at any instant, the charge distribution is not perfectly symmetric: there will be regions of partial positive charge and partial negative charge. This transient asymmetry in the electronic charge around an atom acts through electrostatic interactions to induce a complementary asymmetry in the electron distribution around its neighboring atoms. The resulting attraction between two atoms increases as they come closer to each other, until they are separated by the van der Waals contact distance, which corresponds to 3 to 4 Å, depending on the participating atoms (Figure 2.6). At a shorter distance, very strong repulsive forces become dominant because the outer electron clouds overlap. Energies associated with van der Waals interactions are quite small; typical interactions contribute from 2 to 4 kJ mol−1 (from 0.5 to 1.0 kcal mol−1) per atom pair. However, when the surfaces of two large molecules with complementary shapes come together, a large number of atoms are in van der Waals contact, and the net effect, summed over many atom pairs, can be substantial. Although the motto of all weak electrostatic interactions might be “stability in numbers,” the motto especially applies to van der Waals interactions.
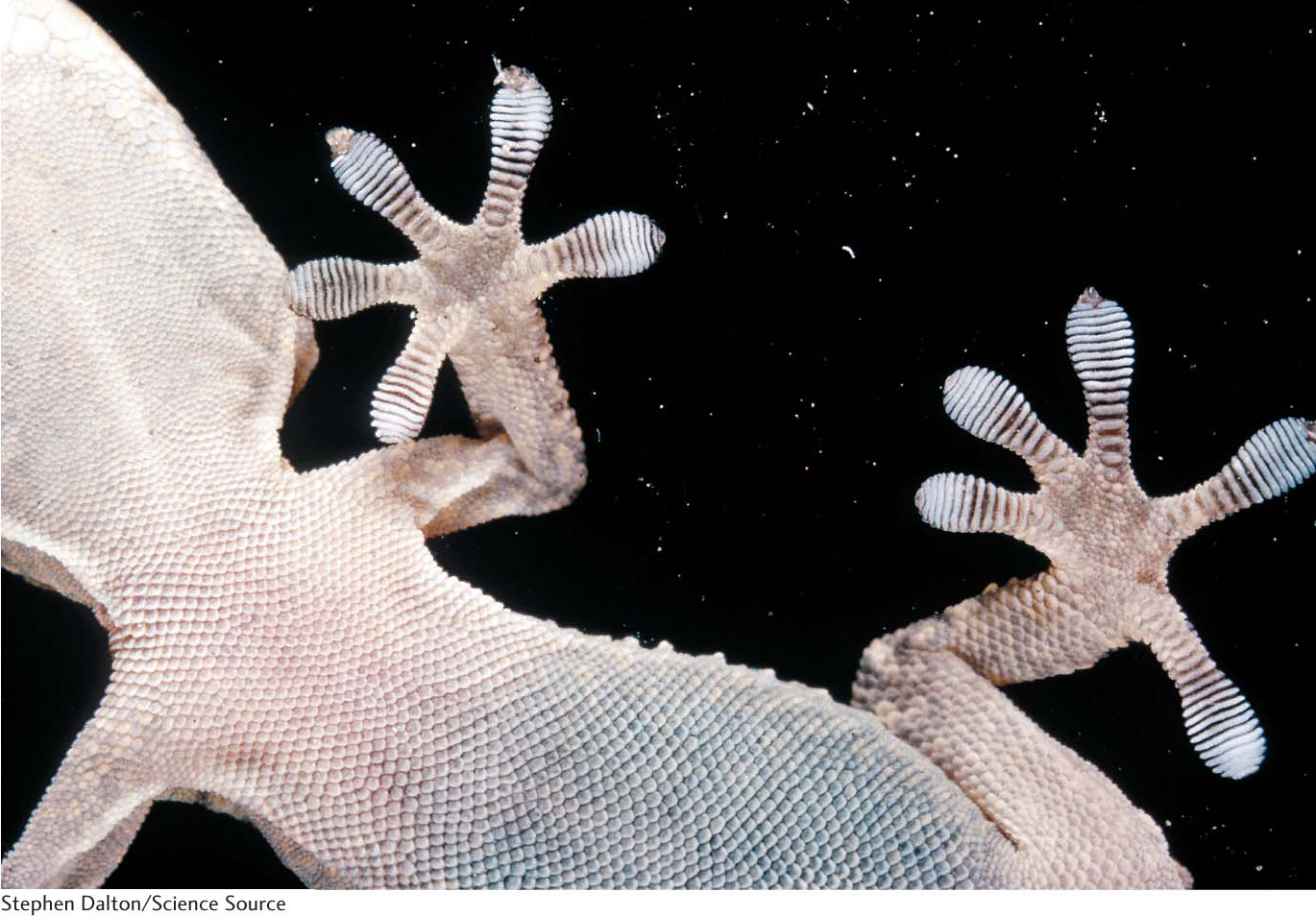
A remarkable example of the power of van der Waals interactions is provided by geckos (Figure 2.7). These creatures can walk up walls and across ceilings, defying gravity because of the van der Waals interactions between their feet and the surface of the wall or ceiling.
22
Weak Bonds Permit Repeated Interactions
!quickquiz! QUICK QUIZ 1
All weak interactions can be said to be fundamentally electrostatic interactions. Explain.
An important feature of weak bonds is that they can be easily broken. DNA provides an excellent example of why the breakage of weak bonds is essential. Hydrogen bonds between base pairs stabilize the double helix and keep the coding information—