30.1 Nitrogen Removal Is the First Step in the Degradation of Amino Acids
✓ 1 Describe the fate of nitrogen that is removed when amino acids are used as fuels.
As already mentioned, amino acids are not stockpiled for use later. Any amino acids not needed as building blocks are degraded to various compounds, depending on the type of amino acid and the tissue from which it originates. The major site of amino acid degradation in mammals is the liver. The first step is the removal of nitrogen. The resulting α-ketoacids are then metabolized so that the carbon skeletons can enter the metabolic mainstream as precursors of glucose or of citric acid cycle intermediates. We will consider the fate of the α-amino group first, followed by that of the carbon skeleton.
Alpha-Amino Groups Are Converted into Ammonium Ions by the Oxidative Deamination of Glutamate
The α-amino group of many amino acids is transferred to α-ketoglutarate to form glutamate, which is then oxidatively deaminated to yield ammonium ion (NH4+):
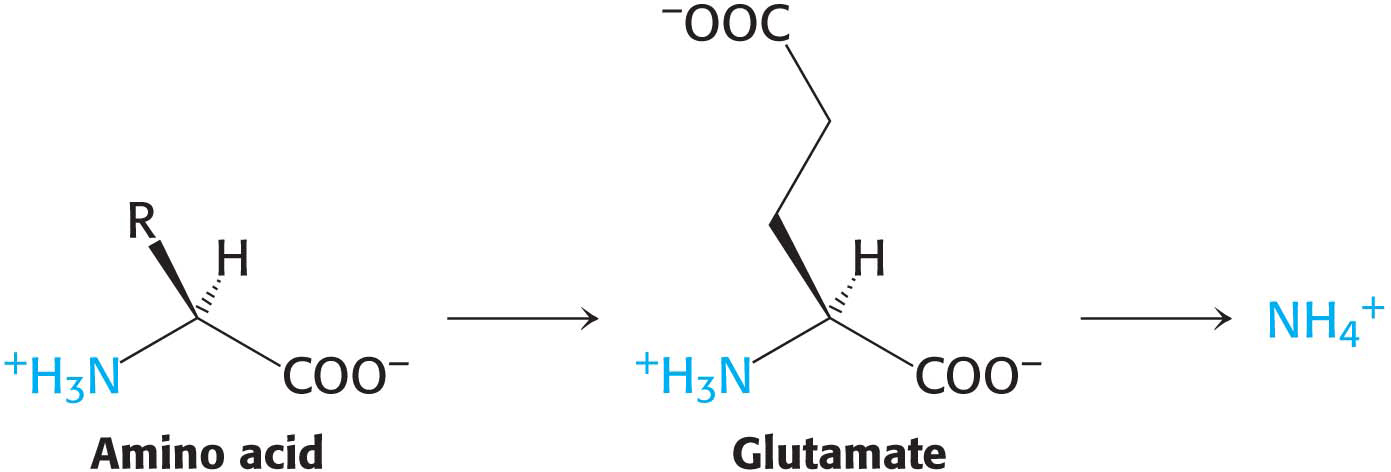
Aminotransferases catalyze the transfer of an α-amino group from an α-amino acid to an α-ketoacid. These enzymes, also called transaminases, generally funnel α-amino groups from a variety of amino acids to α-ketoglutarate for conversion into NH4+. Note that these very same transaminase enzymes can be used in the synthesis of amino acids.

Aspartate aminotransferase, one of the most important of these enzymes, catalyzes the transfer of the α-amino group of aspartate to α-ketoglutarate:

553
Alanine aminotransferase catalyzes the transfer of the amino group of alanine to α-ketoglutarate:
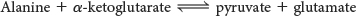
The nitrogen atom that is transferred to α-ketoglutarate in the transamination reaction is converted into free ammonium ion by the oxidative deamination of glutamate, regenerating α-ketoglutarate:
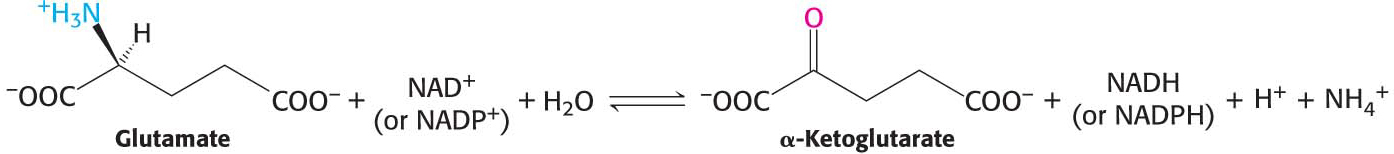
This reaction is catalyzed by glutamate dehydrogenase, an enzyme that we will encounter again in the context of the incorporation of ammonia into biomolecules (Chapter 31). This reaction equilibrium constant is close to 1 in the liver, so the direction of the reaction is determined by the concentrations of reactants and products. Normally, the reaction is driven forward by the rapid removal of ammonium ion. Glutamate dehydrogenase is unusual in that it can use either NADH or NADPH as reducing power. The dehydrogenase, a liver-
In mammals, but not in other organisms, glutamate dehydrogenase is allosterically inhibited by GTP, and stimulated by ADP. These nucleotides exert their regulatory effects in a unique manner. An abortive complex is formed on the enzyme when a product is replaced by substrate before the reaction is complete. For instance, the enzyme bound to glutamate and NAD(P)H is an abortive complex. GTP facilitates the formation of such complexes while ADP destabilizes the complexes.
The sum of the reactions catalyzed by aminotransferases and glutamate dehydrogenase is
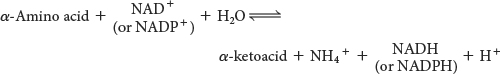
!clinic! CLINICAL INSIGHT: Blood Levels of Aminotransferases Serve a Diagnostic Function
The presence of alanine and aspartate aminotransferase in the blood is an indication of liver damage. Liver damage can occur for a number of reasons, including viral hepatitis, long-
Serine and Threonine Can Be Directly Deaminated
Although the nitrogen atoms of most amino acids are transferred to α-ketoglutarate before their removal, the α-amino groups of serine and threonine can be directly converted into NH4+. These direct deaminations are catalyzed by serine dehydratase and threonine dehydratase, in which pyridoxal phosphate (PLP) is the prosthetic group.
554
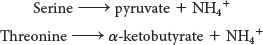
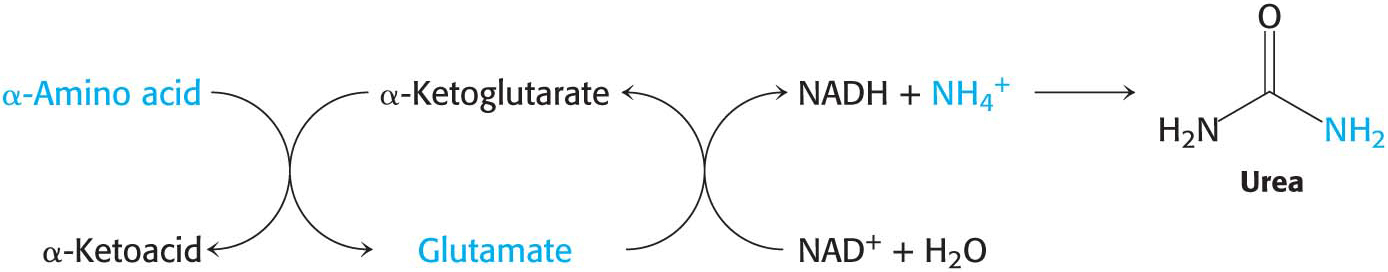
In most terrestrial vertebrates, NH4+ is then converted into urea for excretion, a process that we will consider shortly:
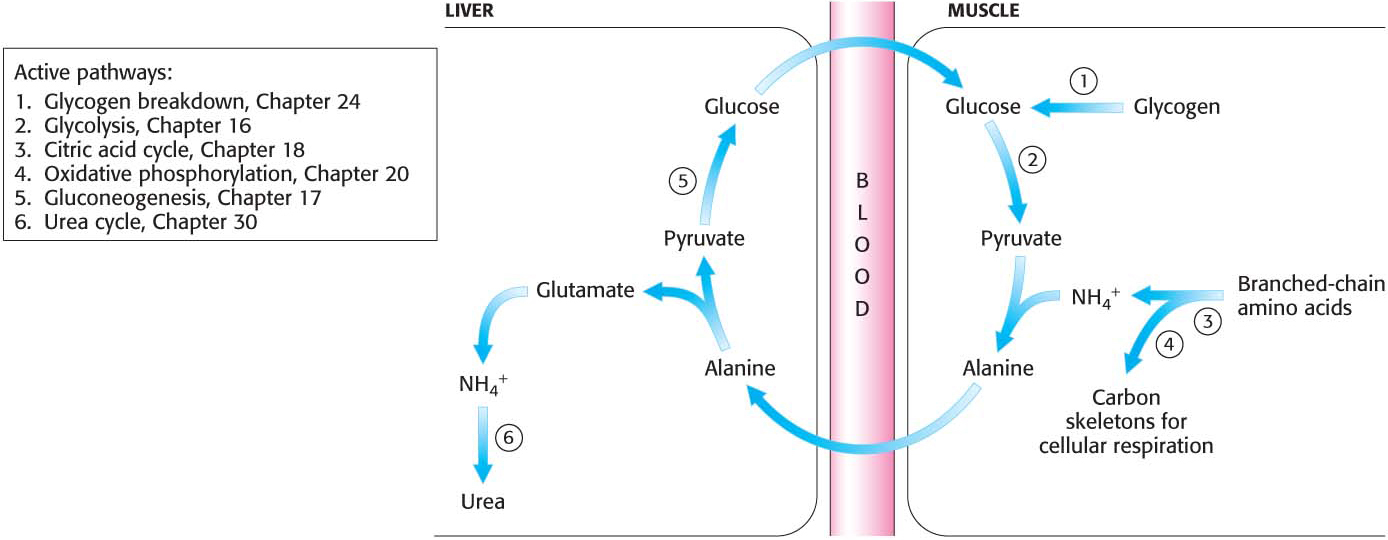
Peripheral Tissues Transport Nitrogen to the Liver
Although much amino acid degradation takes place in the liver, the liver cannot deaminate the branched-
Nitrogen is transported from muscle to the liver in two principal transport forms: alanine and glutamine. Glutamate is formed by transamination reactions, but the nitrogen is then transferred to pyruvate to form alanine, which is released into the blood (Figure 30.1). The liver takes up the alanine and converts it back into pyruvate by transamination. The pyruvate can be used for gluconeogenesis, and the amino group eventually appears as urea. This transport is referred to as the glucose–
555
Glutamine is also a key transport form of nitrogen. Glutamine synthetase catalyzes the synthesis of glutamine from glutamate and NH4+ in an ATP-
!quickquiz! QUICK QUIZ 1
How do aminotransferases and glutamate dehydrogenase cooperate in the metabolism of the α-amino group of amino acids?
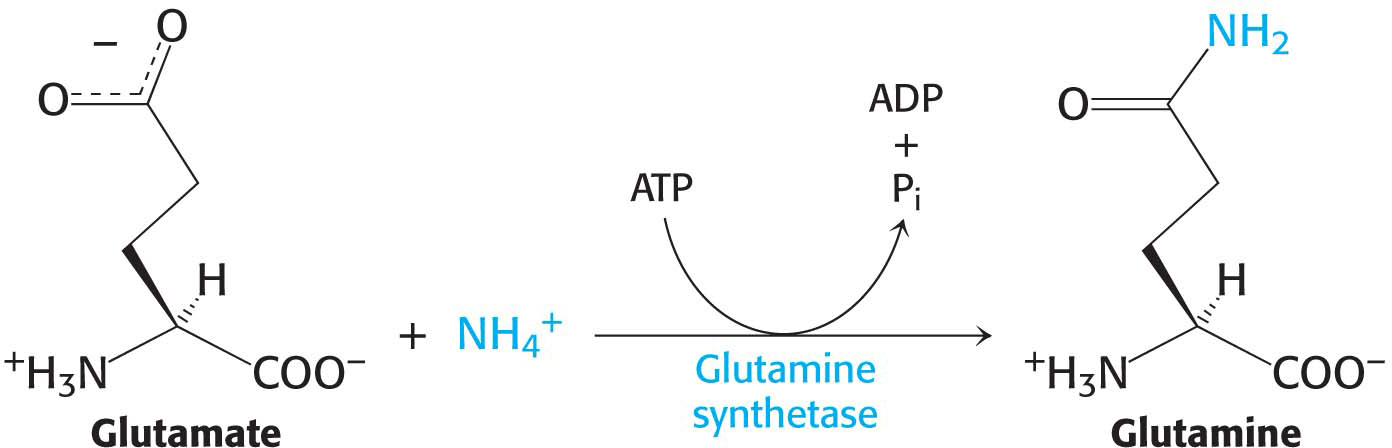
The nitrogen atoms of glutamine can be converted into urea in the liver.