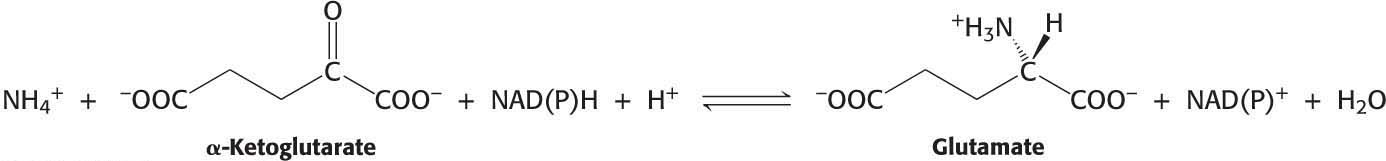31.1 The Nitrogenase Complex Fixes Nitrogen
✓ 3 Explain the centrality of nitrogen fixation to life, and describe how atmospheric nitrogen is converted into a biologically useful form of nitrogen.
To meet the kinetic challenge of nitrogen fixation, nitrogen-fixing organisms employ a complex enzyme with multiple oxidation–reduction centers that enable it to reduce the inactive N2 gas. The nitrogenase complex, which carries out this fundamental transformation, consists of two proteins: a reductase, which provides electrons with high reducing power, and nitrogenase, which uses these electrons to reduce N2 to NH3. The transfer of electrons from the reductase to the nitrogenase component requires the hydrolysis of ATP by the reductase (Figure 31.2).
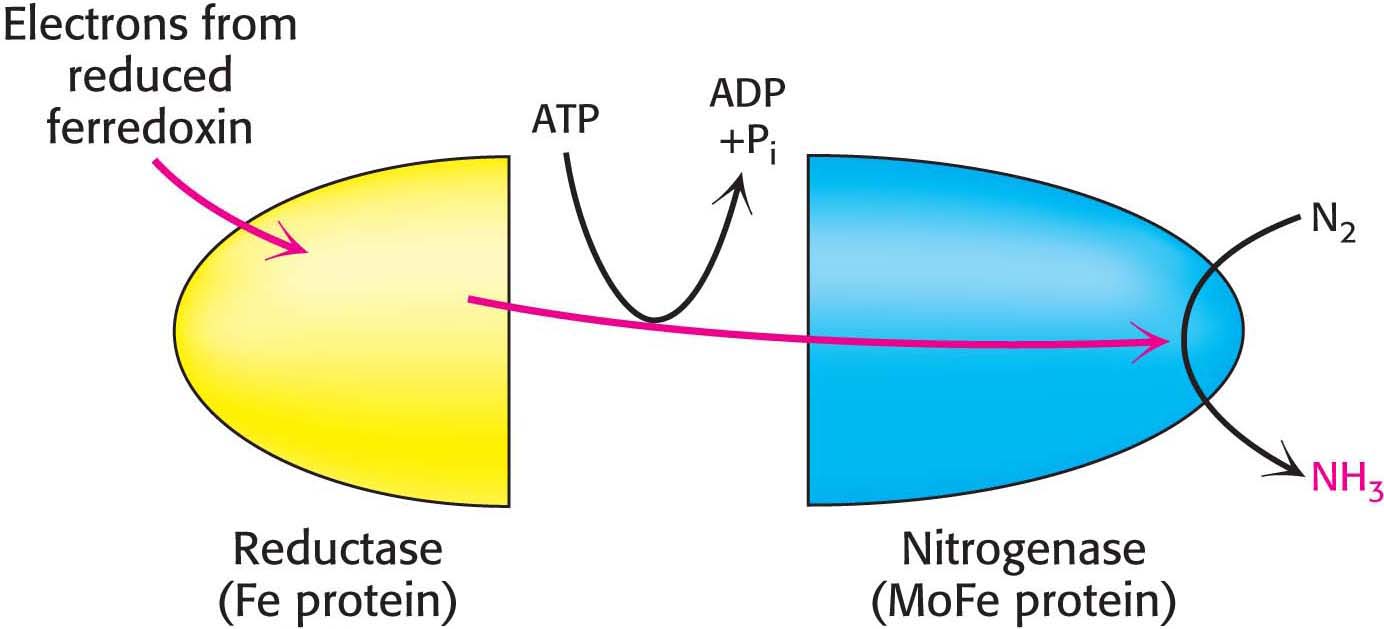
Figure 31.2: Nitrogen fixation. Electrons flow from ferredoxin to the reductase (iron protein, or Fe protein) to nitrogenase (molybdenum–iron protein, or MoFe protein) to reduce nitrogen to ammonia. ATP hydrolysis within the reductase drives conformational changes necessary for the efficient transfer of electrons.
In principle, the reduction of N2 to NH3 is a six-electron process:
However, the biological reaction always generates at least 1 mol of H2 in addition to 2 mol of NH3 for each mole of N ≡ N. Hence, an input of two additional electrons is required:
In most nitrogen-fixing microorganisms, the eight high-potential electrons come from reduced ferredoxin. Two molecules of ATP are hydrolyzed for each electron transferred. Thus, at least 16 molecules of ATP are hydrolyzed for each molecule of N2 reduced:
The product of the reduction, NH3, is a base in aqueous solutions, attracting a proton to form NH4+.
Note that O2 is required for oxidative phosphorylation to generate the ATP necessary for nitrogen fixation. However, the nitrogenase complex is exquisitely sensitive to inactivation by O2. To allow ATP synthesis and nitrogenase to function simultaneously, leguminous plants maintain a very low concentration of free O2 in their root nodules, the location of the nitrogenase. This is accomplished by binding O2 to leghemoglobin, a homolog of hemoglobin.
The Molybdenum–Iron Cofactor of Nitrogenase Binds and Reduces Atmospheric Nitrogen
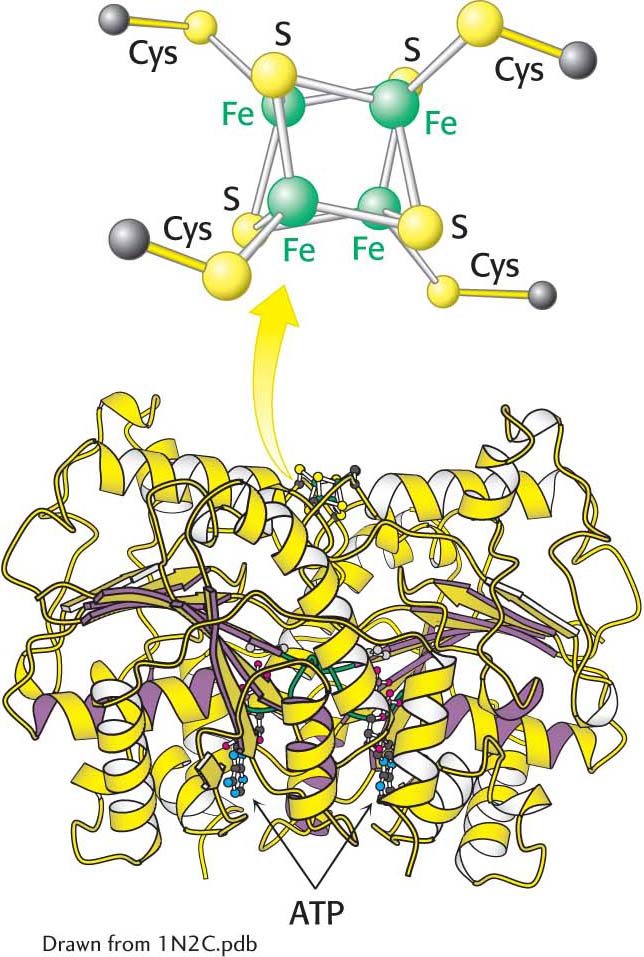
Figure 31.3:  The Fe protein. This protein is a dimer composed of two polypeptide chains linked by a 4Fe-4S cluster.
The Fe protein. This protein is a dimer composed of two polypeptide chains linked by a 4Fe-4S cluster.
Both the reductase and the nitrogenase components of the complex are iron–sulfur proteins, a type of electron carrier that we have seen many times in our study of biochemistry—for instance, in the electron-transport chains of oxidative phosphorylation and photosynthesis. The reductase (also called the iron protein or the Fe protein) is a dimer of identical 30-kDa subunits bridged by a 4Fe-4S cluster (Figure 31.3). The role of the reductase is to transfer electrons from a suitable donor, such as reduced ferredoxin, to the nitrogenase component.
The nitrogenase component, an α2β2 tetramer (240 kDa), requires the FeMo cofactor, which consists of [Fe4-S3] and [Mo-Fe3-S3] subclusters joined by three disulfide bonds. A carbon atom (the interstitial carbon) sits at the interstices of the iron atoms of the FeMo cofactor. The FeMo cofactor is also coordinated to a homocitrate moiety and to the α subunit through one histidine residue and one cysteinate residue (Figure 31.4).
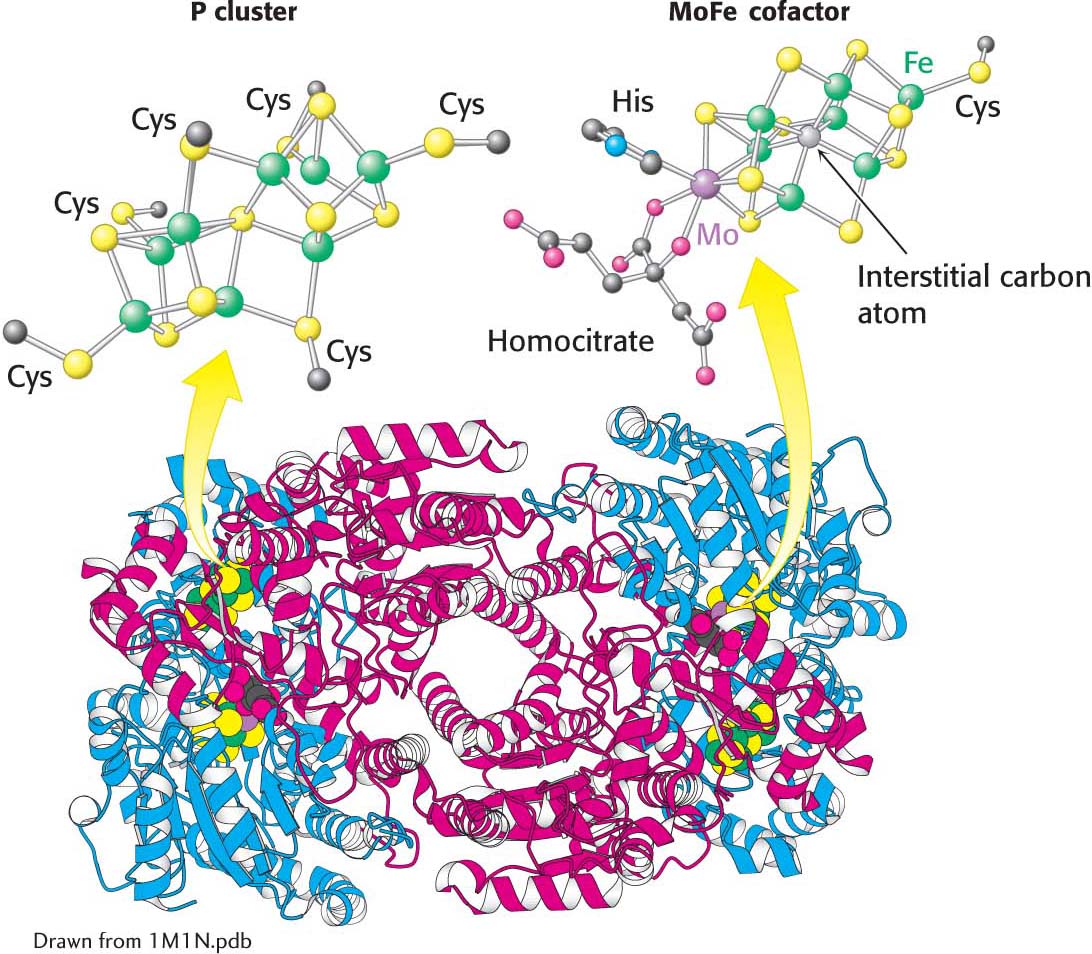
Figure 31.4: The MoFe protein. This protein is a heterotetramer composed of two α subunits (red) and two β subunits (blue). Notice that the protein contains two copies each of two types of clusters: P clusters and MoFe cofactors. Each P cluster contains eight iron atoms (green) and seven sulfides linked to the protein by six cysteinate residues. Each MoFe cofactor contains one molybdenum atom, seven iron atoms, nine sulfides, a central atom, and a homocitrate and is linked to the protein by one cysteinate residue and one histidine residue.
Electrons flow from the reductase to the nitrogenase at a part of the nitrogenase called the P cluster, an iron- and sulfur-rich site. From there, the electrons progress to the FeMo cofactor, the redox center in the nitrogenase. Because molybdenum is present in this cluster, the nitrogenase component is also called the molybdenum–iron protein or MoFe protein. The MoFe cofactor is the site of nitrogen fixation—the conversion of N2 into ammonia.
Ammonium Ion Is Incorporated into an Amino Acid Through Glutamate and Glutamine
The next task in the assimilation of nitrogen into biomolecules is to incorporate ammonium ion (NH4+) into the biochemically versatile amino acids. α-Ketoglutarate and glutamate play pivotal roles as the acceptor of ammonium ion, forming glutamate and glutamine, respectively. Glutamate is synthesized from NH4+ and α-ketoglutarate, a citric acid cycle intermediate, by the action of glutamate dehydrogenase:
DID YOU KNOW?
Amidation describes a reaction in which an amine reacts with a carboxylic acid to yield an amide.
Recall that glutamate dehydrogenase also plays a key role in the removal of nitrogen from biological systems.
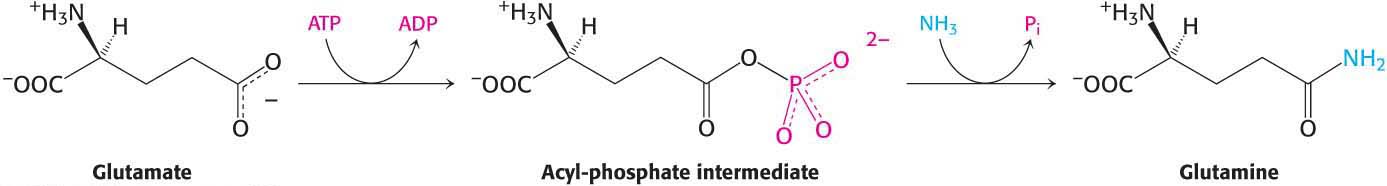
A second ammonium ion is incorporated into glutamate to form glutamine by the action of glutamine synthetase. This amidation is driven by the hydrolysis of ATP:
!quickquiz! QUICK QUIZ 1
Trace the flow of nitrogen from atmospheric N2 to glutamine.
ATP participates directly in the reaction by phosphorylating the side chain of glutamate to form an acyl-phosphate intermediate, which then reacts with ammonia to form glutamine.
Glutamate subsequently donates its α-amino group to various ketoacids by transamination reactions to form most of the amino acids. Glutamine, the other major nitrogen donor, contributes its side-chain nitrogen atom in the biosynthesis of a wide range of important compounds.

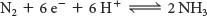
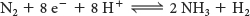
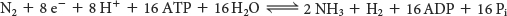

 The Fe protein. This protein is a dimer composed of two polypeptide chains linked by a 4Fe-
The Fe protein. This protein is a dimer composed of two polypeptide chains linked by a 4Fe-