32.3 The Purine Ring Is Assembled on Ribose Phosphate
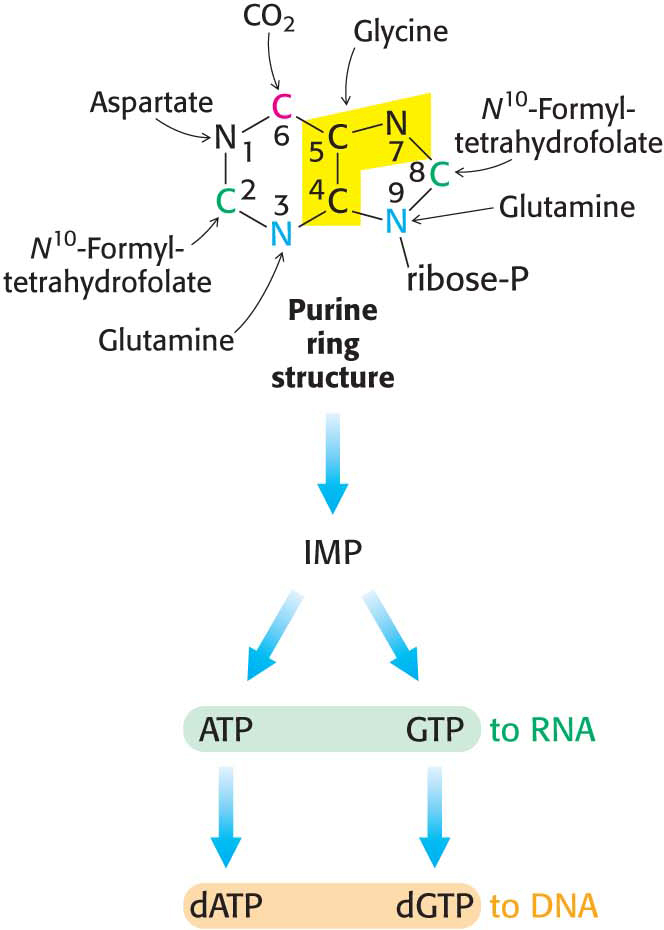
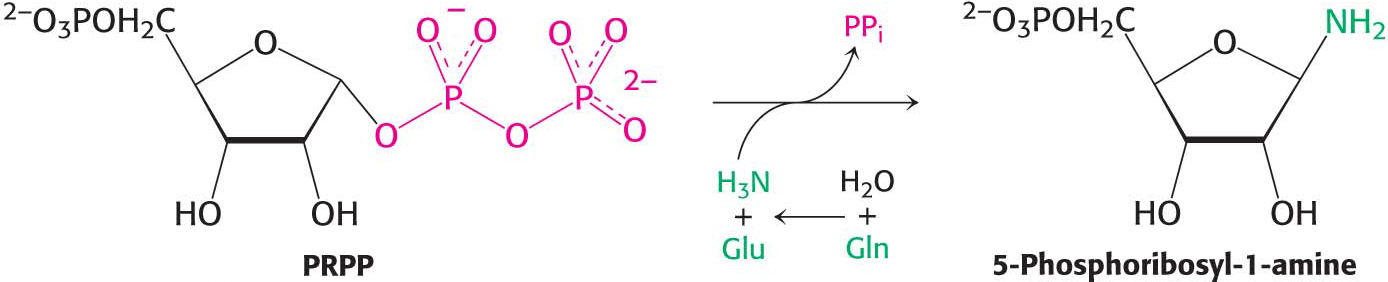
Purines, like pyrimidines, are synthesized de novo, beginning with simple starting materials (Figure 32.4). In contrast with pyrimidine synthesis, the first step in purine assembly begins with the attachment to ribose. De novo purine biosynthesis, like pyrimidine biosynthesis, requires PRPP, but, for purines, PRPP provides the foundation on which the bases are constructed step by step. The initial step is the displacement of the pyrophosphate of PRPP by ammonia, rather than by a preassembled base, to produce 5-
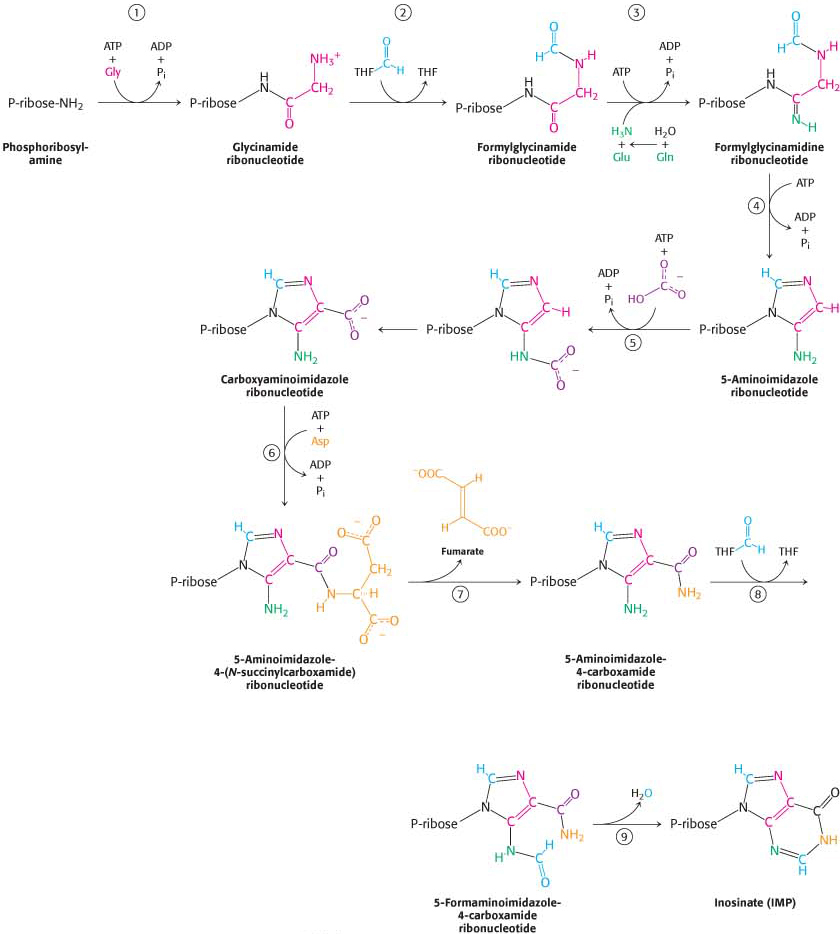
Nine additional steps are required to assemble the purine ring. De novo purine biosynthesis proceeds by successive steps of activation by phosphorylation followed by displacement, as shown in (Figure 32.5). The final product is the nucleotide inosine monophosphate (IMP, or inosinate). The amino acids glycine, glutamine, and aspartate are required precursors. The synthesis of purines depends on tetrahydrofolate, a prominent carrier of activated one-
AMP and GMP Are Formed from IMP
DID YOU KNOW?
In the ring form of ribose sugars, the β configuration means that the group attached at C-
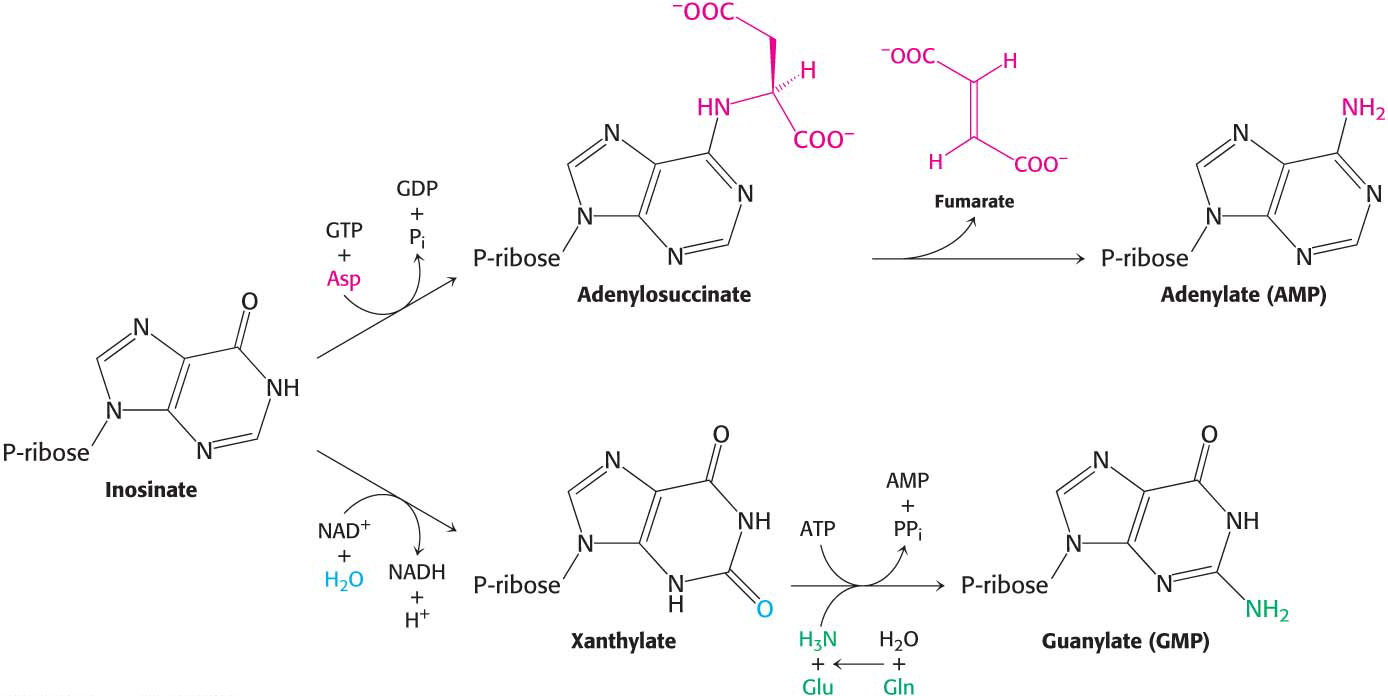
Inosinate, although a component of some RNA molecules, serves primarily as a precursor to the other purines. Inosinate is at the base of a branched pathway that leads to both AMP and GMP (Figure 32.6). Adenylate is synthesized from inosinate by the substitution of an amino group for the carbonyl oxygen atom at C-
!quickquiz! QUICK QUIZ 2
Identify the sources of all of the atoms in a purine ring.
Guanosine monophosphate (GMP, or guanylate) is synthesized by the oxidation of inosinate to xanthine monophosphate (XMP, or xanthylate), followed by the incorporation of an amino group at C-
591
592
!bio! BIOLOGICAL INSIGHT: Enzymes of the Purine-Synthesis Pathway Are Associated with One Another in Vivo
Biochemists believe that the enzymes of many metabolic pathways, such as glycolysis and the citric acid cycle, are physically associated with one another. Such associations would increase the efficiency of pathways by facilitating the movement of the product of one enzyme to the active site of the next enzyme in the pathway. The evidence for such associations comes primarily from experiments in which one component of a pathway, carefully isolated from the cell, is found to be bound to other components of the pathway. However, these observations raise the question, do enzymes associate with one another in vivo or do they spuriously associate during the isolation procedure? Recent in vivo evidence shows that the enzymes of the purine-

593
Bases Can Be Recycled by Salvage Pathways
Purine nucleotides, like pyrimidine nucleotides, can also be synthesized by salvaging and recycling intact purines released by the hydrolytic degradation of nucleic acids and nucleotides. Such salvage pathways skip most of the energy-
Two salvage enzymes with different specificities recover purine bases. Adenine phosphoribosyltransferase catalyzes the formation of adenylate,

whereas hypoxanthine-
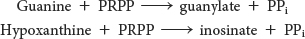
Purine salvage pathways are especially noteworthy in light of the amazing consequences of their absence.