39.2 Amino Acids Are Activated by Attachment to Transfer RNA
✓ 2 Identify the step in protein synthesis in which translation takes place.
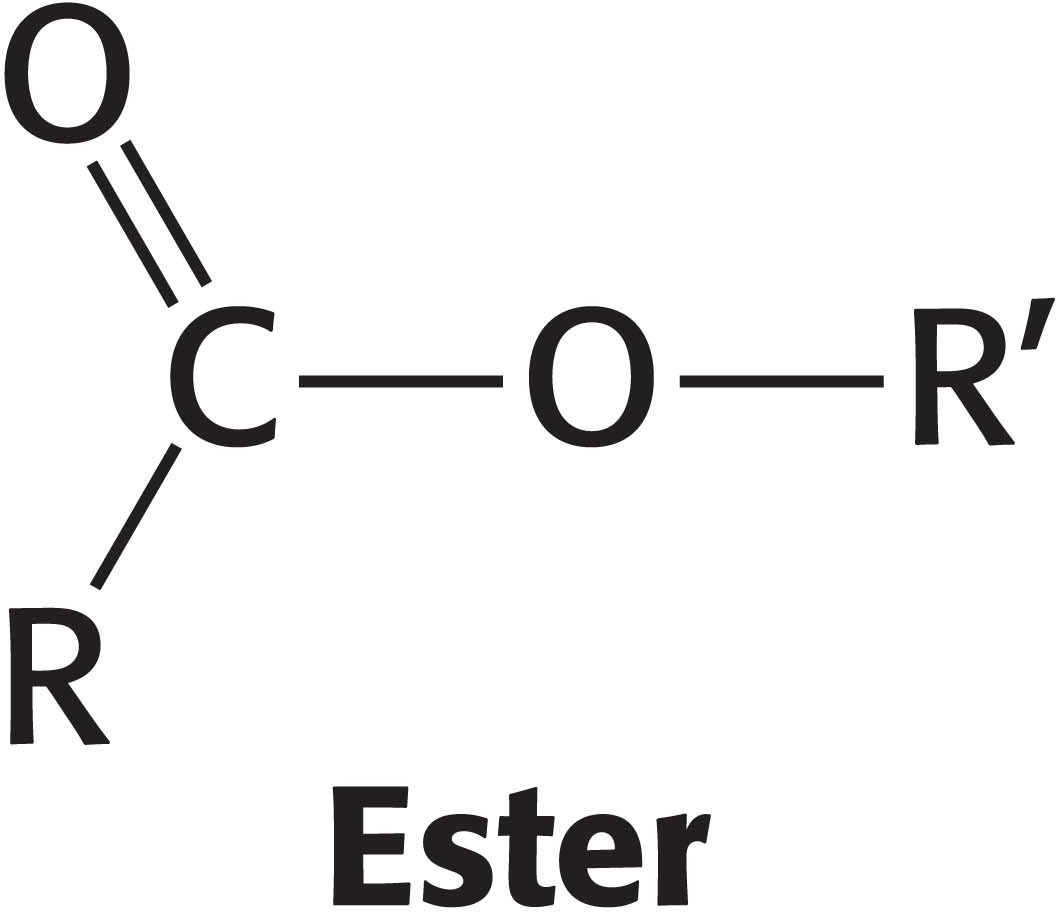
Before codon and anticodon meet, the amino acids required for protein synthesis must first be attached to specific tRNA molecules, linkages that are crucial for two reasons. First, the attachment of a given amino acid to a particular tRNA establishes the genetic code. When an amino acid has been linked to a tRNA, it will be incorporated into a growing polypeptide chain at a position dictated by the anticodon of the tRNA. Second, because the formation of a peptide bond between free amino acids is not thermodynamically favorable, the amino acid must first be activated in order for protein synthesis to proceed. The activated intermediates in protein synthesis are amino acid esters, in which the carboxyl group of an amino acid is linked to either the 2′- or the 3′-hydroxyl group of the ribose unit at the 3′ end of tRNA. An amino acid ester of tRNA is called an aminoacyl-tRNA or sometimes a charged tRNA (Figure 39.3). For a specific amino acid attached to its cognate tRNA—for instance, threonine—the charged tRNA is designated Thr-tRNAThr.
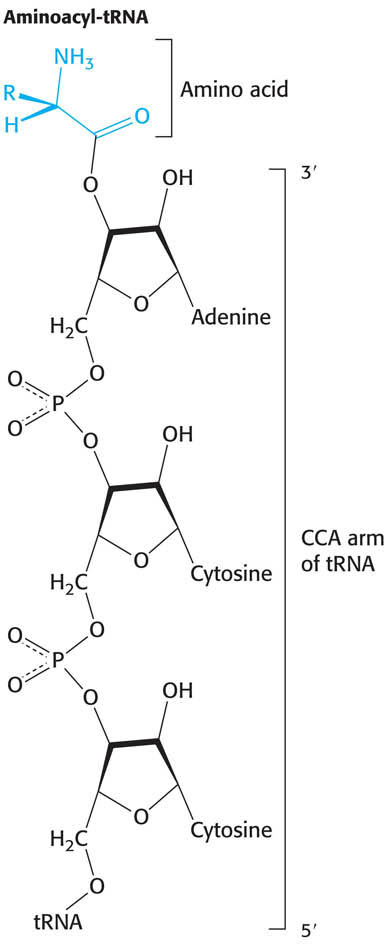
Figure 39.3: Aminoacyl-tRNA. Amino acids are coupled to tRNAs through ester linkages to either the 2′- or the 3′-hydroxyl group of the 3′-adenosine residue. A linkage to the 3′-hydroxyl group is shown.
Amino Acids Are First Activated by Adenylation
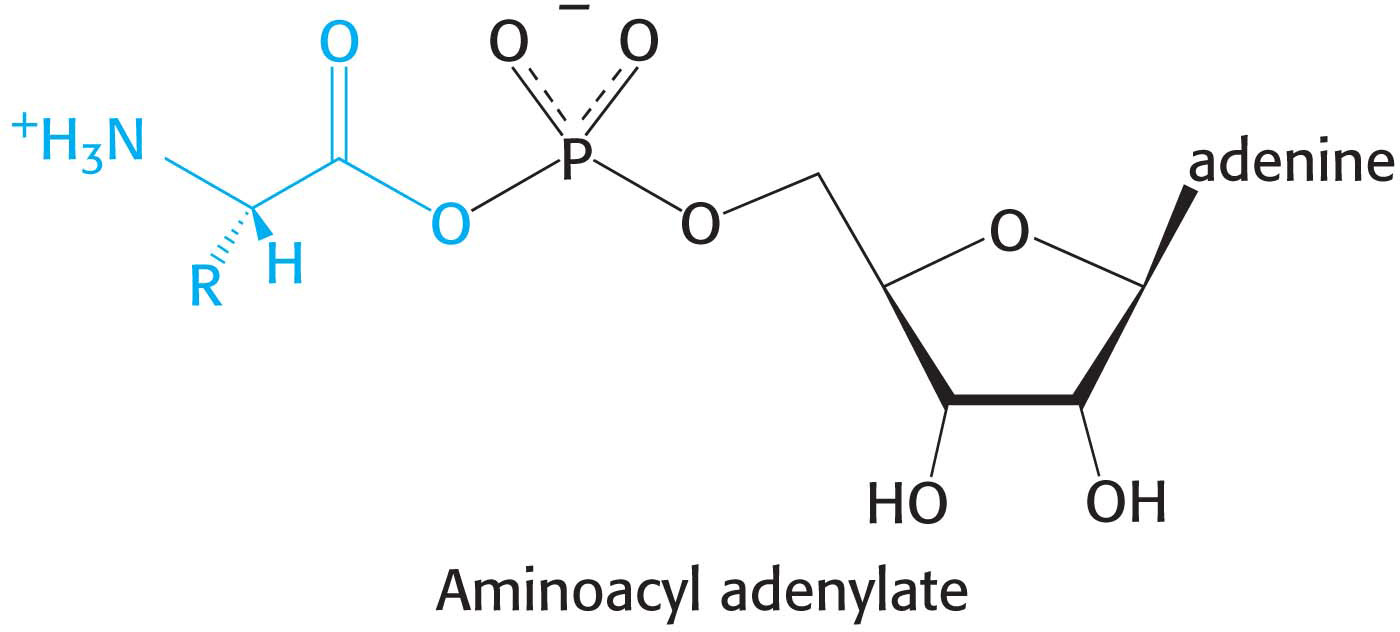
The activation of amino acids is catalyzed by specific aminoacyl-tRNA synthetases. The first step is the formation of an aminoacyl adenylate from an amino acid and ATP. In this molecule, the carboxyl group of the amino acid is linked to the phosphoryl group of AMP; hence, it is also known as aminoacyl-AMP.
The next step is the transfer of the aminoacyl group of aminoacyl-AMP to a particular tRNA molecule to form aminoacyl-tRNA:
The sum of the activation and transfer steps is
The ΔG°′ of this reaction is close to zero because the free energy of hydrolysis of the ester bond of aminoacyl-tRNA is similar to that for the hydrolysis of ATP to AMP and PPi. As we have seen many times, the reaction is driven by the hydrolysis of pyrophosphate. The sum of these three reactions is highly exergonic:
Thus, the equivalent of two molecules of ATP are consumed in the synthesis of each aminoacyl-tRNA.
The activation and transfer steps for a particular amino acid are catalyzed by the same aminoacyl-tRNA synthetase. Indeed, the aminoacyl-AMP intermediate does not dissociate from the synthetase. Rather, it is tightly bound to the active site of the enzyme by noncovalent interactions. Translation takes place with the formation of the ester linkage between an amino acid and a specific tRNA. Thus aminoacyl-tRNA synthetases are the actual translators of the genetic code.
Aminoacyl-tRNA Synthetases Have Highly Discriminating Amino Acid Activation Sites
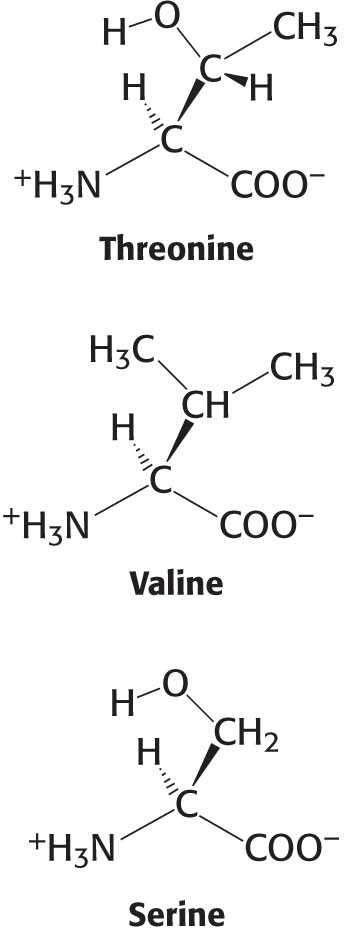
For protein synthesis to be accurate, the correct amino acid must bond to the correct tRNA. Each aminoacyl-tRNA synthetase is highly specific for a given amino acid. Indeed, a synthetase will incorporate the incorrect amino acid only once in 104 or 105 reactions. How is this level of specificity achieved? Each aminoacyl-tRNA synthetase takes advantage of the properties of its amino acid substrate. Let us consider the challenge faced by threonyl-tRNA synthetase, as an example. Threonine is similar to two other amino acids—namely, valine and serine. Valine has almost exactly the same shape as that of threonine, except that it has a methyl group in place of a hydroxyl group. Like threonine, serine has a hydroxyl group but lacks the methyl group. How can the threonyl-tRNA synthetase prevent the coupling of these amino acids to threonyl-tRNA (abbreviated tRNAThr)?
The structure of threonyl-tRNA synthetase’s binding site for the amino acid reveals how coupling with valine is prevented (Figure 39.4). The enzyme’s active site contains a zinc ion. Threonine binds to the zinc ion through its amino group and its side-chain hydroxyl group. The side-chain hydroxyl group is further recognized by an aspartate residue to which it hydrogen bonds. The methyl group present in valine in place of this hydroxyl group cannot participate in these interactions; it will not bind at the active site and, hence, does not become adenylated and transferred to threonyl-tRNA.
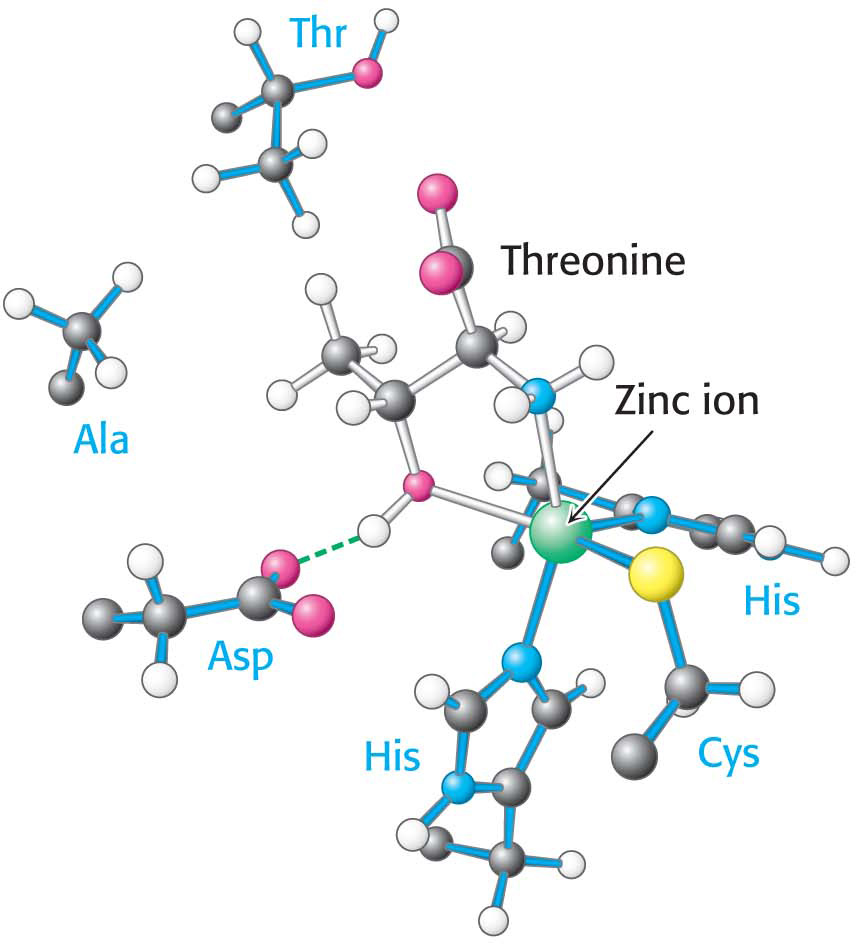
Figure 39.4: The active site of threonyl-tRNA synthetase. Notice that the site for binding the amino acid includes a zinc ion (green ball) that coordinates threonine through its amino and hydroxyl groups.
The zinc site is less well suited to discrimination against serine because this amino acid does have a hydroxyl group that can bind to the zinc. Indeed, with only this mechanism available, threonyl-tRNA synthetase does mistakenly couple serine to threonyl-tRNA at a rate 10−2 to 10−3 times that for threonine. As noted, this error rate is likely to lead to many translation errors. How is the formation of Ser-tRNAThr prevented?
Proofreading by Aminoacyl-tRNA Synthetases Increases the Fidelity of Protein Synthesis
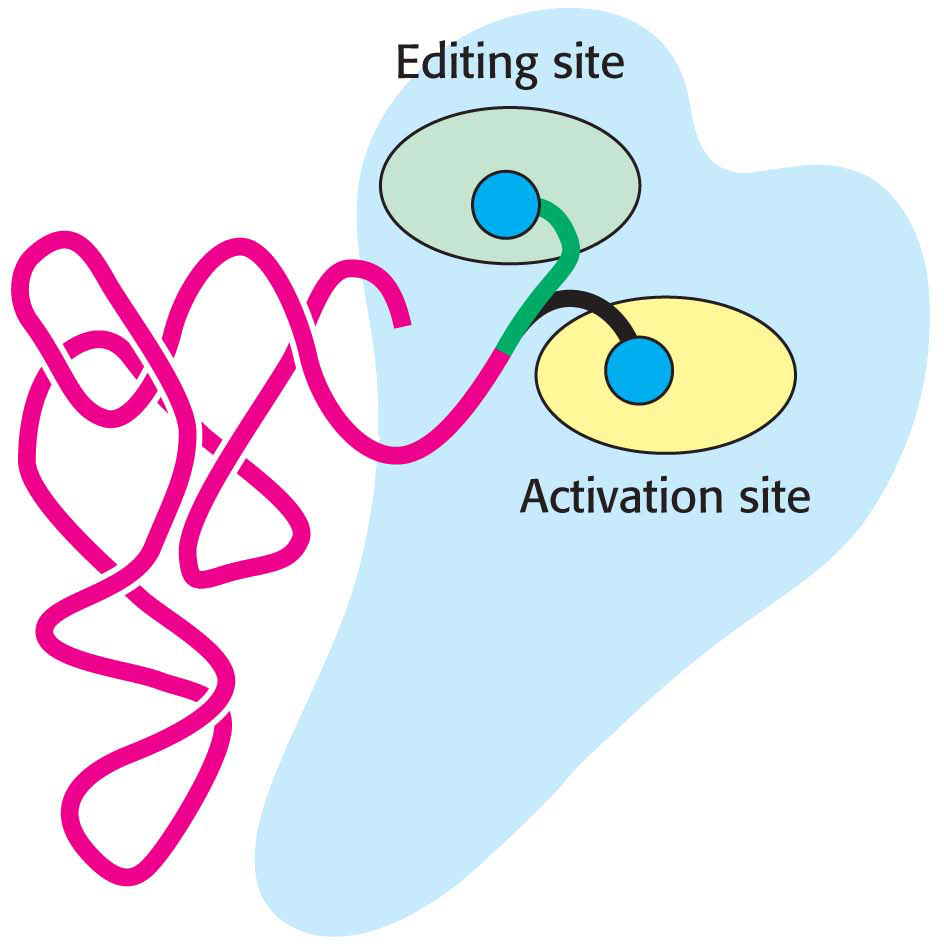
Figure 39.5: The editing of aminoacyl-tRNA. The flexible CCA arm of an aminoacyl-tRNA can move the amino acid between the activation site and the editing site. If the amino acid fits well into the editing site, the amino acid is removed by hydrolysis.
In addition to its active site, threonyl-tRNA synthetase has an editing site located 20 Å from the active site. This site accepts serine attached to tRNAThr and hydrolyzes the bond between serine and tRNAThr, providing the synthetase an opportunity to correct its mistakes and improve its accuracy to less than one mistake in 104. The CCA arm with serine attached can swing out of the activation site and into the editing site (Figure 39.5). Thus, the aminoacyl-tRNA can be edited without dissociating from the synthetase.
What prevents the hydrolysis of Thr-tRNAThr? The discrimination of serine from threonine is relatively easy because threonine contains an extra methyl group, and this methyl group prevents Thr-tRNAThr from fitting into the editing site. Most aminoacyl-tRNA synthetases contain editing sites in addition to acylation sites. These complementary pairs of sites function as a double sieve to ensure very high fidelity. In general, the active site rejects amino acids that are larger than the correct one because there is insufficient room for them, whereas the editing site cleaves activated species that are smaller than the correct one.
Synthetases Recognize the Anticodon Loops and Acceptor Stems of Transfer RNA Molecules
How do synthetases choose their tRNA partners? This enormously important step is the point at which “translation” takes place—at which the correlation between the amino acid and the nucleic acid vocabularies is made. In a sense, aminoacyl-tRNA synthetases are the only molecules in biology that “know” the genetic code. Their precise recognition of tRNAs is as important for high-fidelity protein synthesis as is the accurate selection of amino acids.
A priori, the anticodon of tRNA would seem to be a good identifier of the appropriate tRNA partner for a synthetase because each type of tRNA has a different one. Indeed, some synthetases recognize their tRNA partners primarily on the basis of their anticodons, although they may also recognize other aspects of tRNA structure. The most direct evidence for how recognition takes place comes from crystallographic studies of complexes formed between synthetases and their cognate tRNAs. Figure 39.6 shows the aspects of the tRNA molecule that are recognized by tRNA synthetases. Note that many of the recognition sites are loops rich in unusual bases that can provide structural identifiers.
!quickquiz! QUICK QUIZ 1
Why is the synthesis of aminoacyl-tRNA the crucial step in protein synthesis?
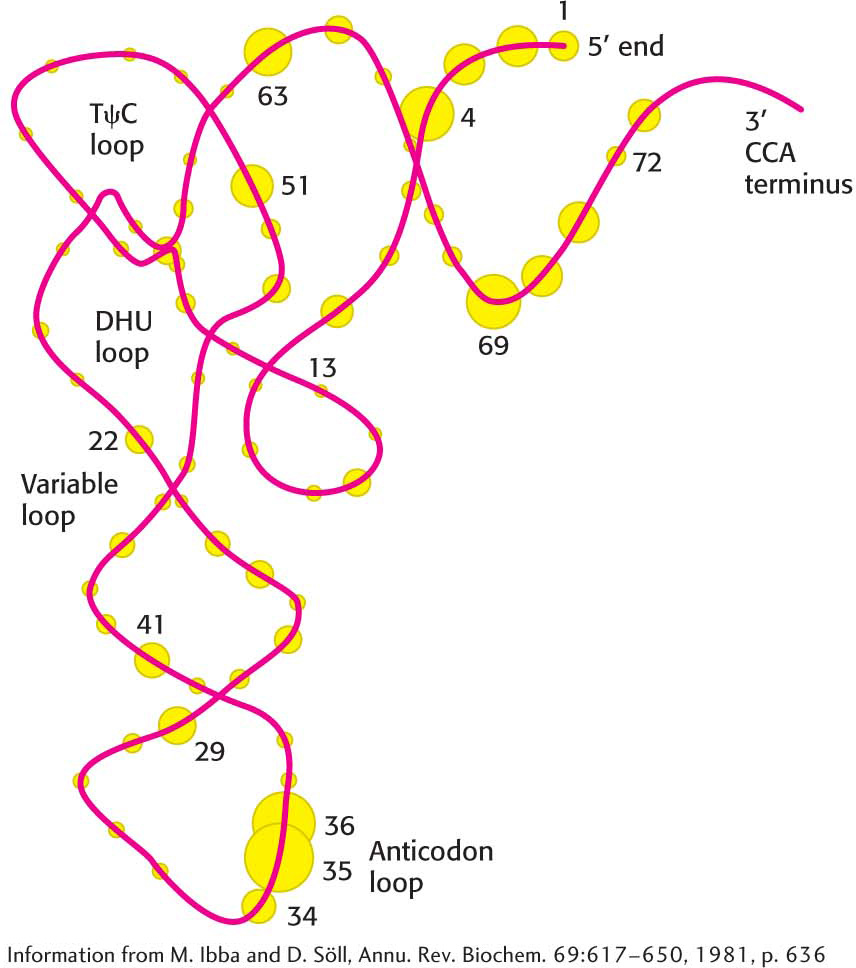
Figure 39.6: Recognition sites on tRNA. Circles represent nucleotides, and the sizes of the circles are proportional to the frequency with which they are used as recognition sites by aminoacyl-tRNA synthetases. The numbers indicate the positions of the nucleotides in the base sequence, beginning from the 5′ end of the tRNA molecule.