5.3 Immunological Techniques Are Used to Purify and Characterize Proteins
✓ 5 Explain how immunological techniques can be used to purify and identify proteins.
For enzymes, the assay is a measure of enzyme activity—the disappearance of substrate or the appearance of product. Let us examine the purification of another type of protein, the estrogen receptor. In so doing, we will learn several more biochemical characterization techniques and we will see the power of immunological techniques.
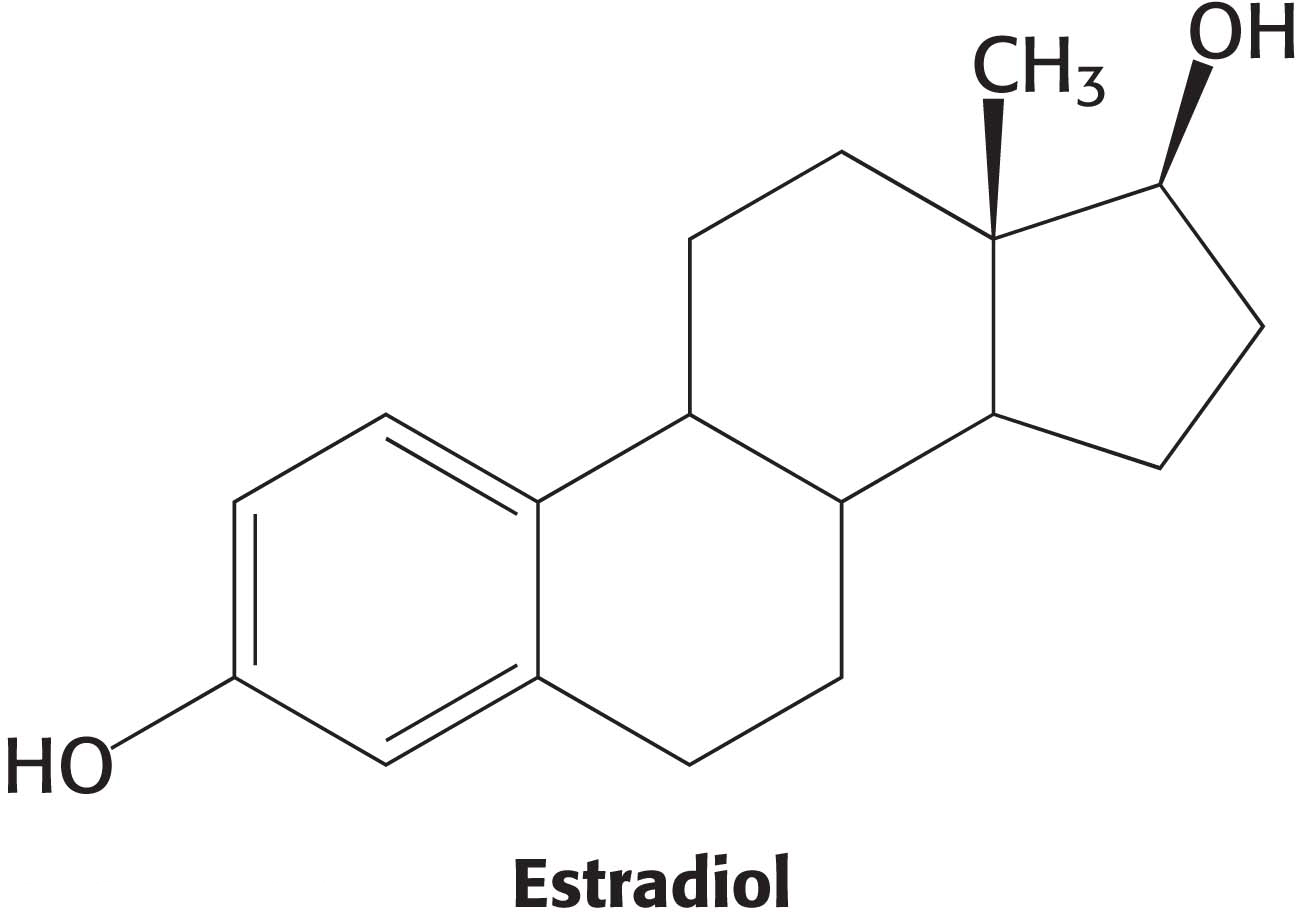
The estrogen-receptor protein binds the female steroid hormone estradiol, an estrogen, and then regulates the expression of genes that play a role in the development of the female phenotype. But the estrogen receptor has no enzyme activity: How can we test for its presence? We can approach this question by asking another one: What is the most distinctive property of the estrogen receptor? The estrogen receptor is the only protein in estrogen-responsive tissues such as rat uterus that can bind to the estradiol with high affinity. We can exploit this distinctive property by exposing the cytosol (Figure 5.1) from rat uteri, which contains the receptor, to radiolabeled estradiol. Because the estrogen receptor has such a high affinity for estradiol, it will be the only protein in the cell that binds to this radioactive steroid. How do we know that the receptor has bound to this steroid? The answer to this question requires a second part of our assay—a means to detect the estradiol–receptor complex. A technique called zonal, density gradient, or, more commonly, gradient centrifugation provides a convenient means of detection.
Centrifugation Is a Means of Separating Proteins
Earlier, we examined the technique of differential centrifugation, which is used to fractionate the cell into several components consisting of different organelles. Here, we examine ultracentrifugation, which is capable of separating much smaller molecular complexes. Proteins or protein complexes will move in a liquid medium when subjected to a centrifugal force. The rate at which these complexes or particles move when exposed to such a force is determined by three key characteristics: mass, density, and shape. A convenient means of quantifying the rate of movement is to calculate the sedimentation coefficient(s) of a particle by using the following equation:
where m is the mass of the particle, 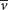 is the partial specific volume (the reciprocal of the particle density), ρ is the density of the medium, and f is the frictional coefficient (a measure of the shape of the particle). The
is the partial specific volume (the reciprocal of the particle density), ρ is the density of the medium, and f is the frictional coefficient (a measure of the shape of the particle). The 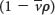 term is the buoyant force exerted by the liquid medium.
term is the buoyant force exerted by the liquid medium.
Sedimentation coefficients are usually expressed in Svedberg units (S), equal to 10−13 s. The smaller the S value, the more slowly a molecule moves in a centrifugal field. The S values for a number of proteins are listed in Table 5.2.
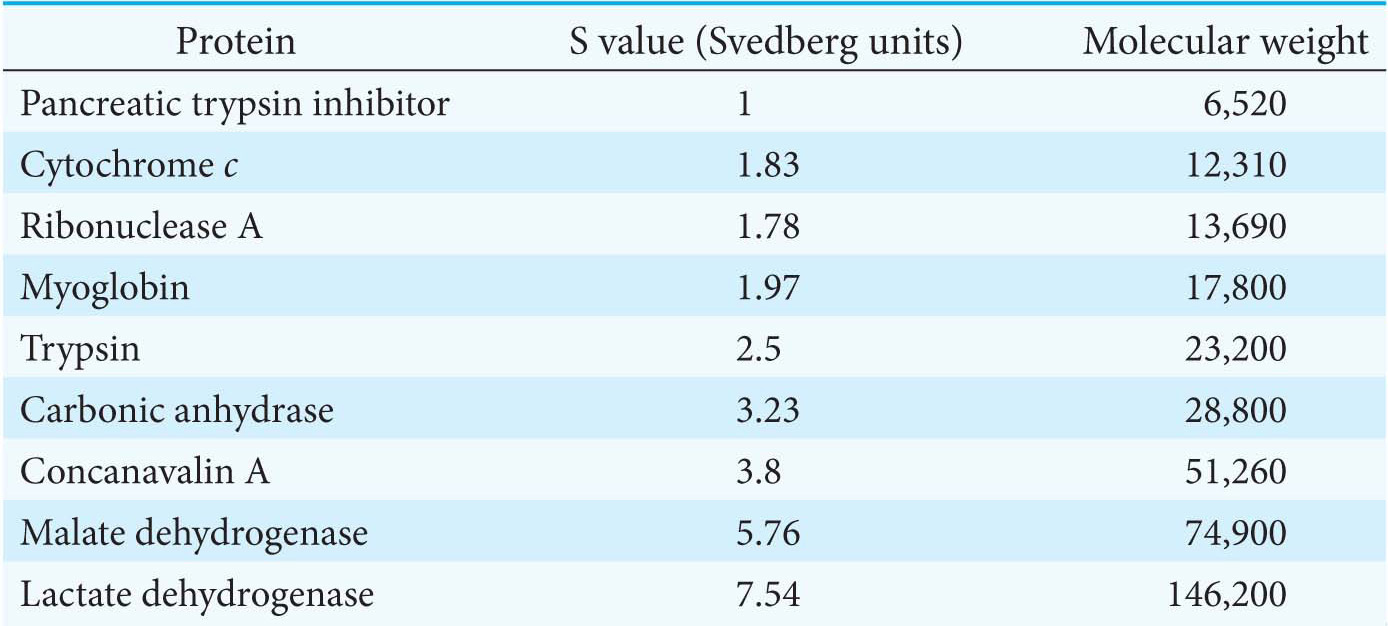
Table 5.2 S values and molecular weights of sample proteins
Several important conclusions can be drawn from the preceding equation:
The sedimentation velocity of a particle depends in part on its mass. A more massive particle sediments more rapidly than does a less massive particle of the same shape and density.
Shape, too, influences the sedimentation velocity because it affects the viscous drag. The frictional coefficient f of a compact particle is smaller than that of an extended particle of the same mass. Hence, elongated particles sediment more slowly than do spherical ones of the same mass.
A dense particle moves more rapidly than does a less dense one because the opposing buoyant force 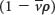 is smaller for the denser particle.
is smaller for the denser particle.
-
Gradient Centrifugation Provides an Assay for the Estradiol–Receptor Complex
How do we analyze the rate of a protein’s movement in a centrifugal field? The first step is to form a density gradient in a centrifuge tube. Differing proportions of a low-density solution (such as 5% sucrose) and a high-density solution (such as 20% sucrose) are mixed to create a linear gradient of sucrose concentration ranging from 20% at the bottom of the tube to 5% at the top (Figure 5.14). The role of the gradient is twofold. First, the gradient stabilizes the liquid and prevents movement of the liquid due to convection. Second, the resistance to movement resulting from the increasing density of the gradient helps to improve separation of the sample components.
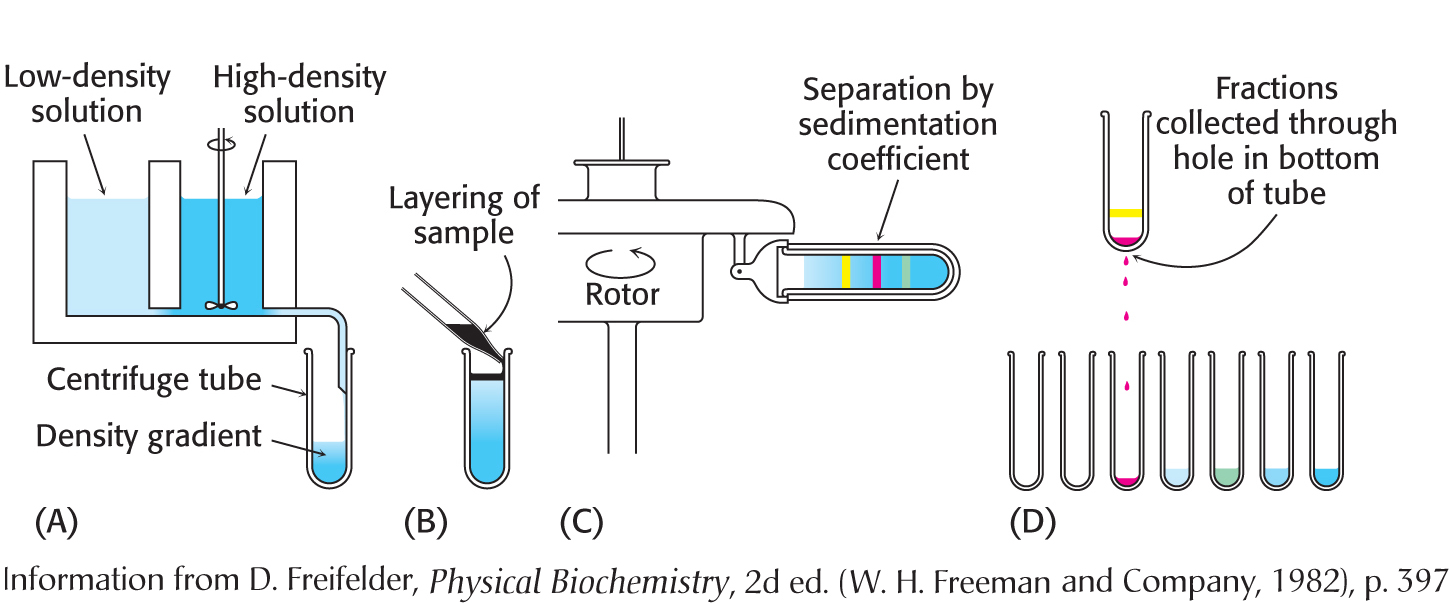
Figure 5.14: Zonal centrifugation. The steps are as follows: (A) form a density gradient, (B) layer the sample on top of the gradient, (C) place the tube in a swinging-bucket rotor and centrifuge it, and (D) collect the samples.
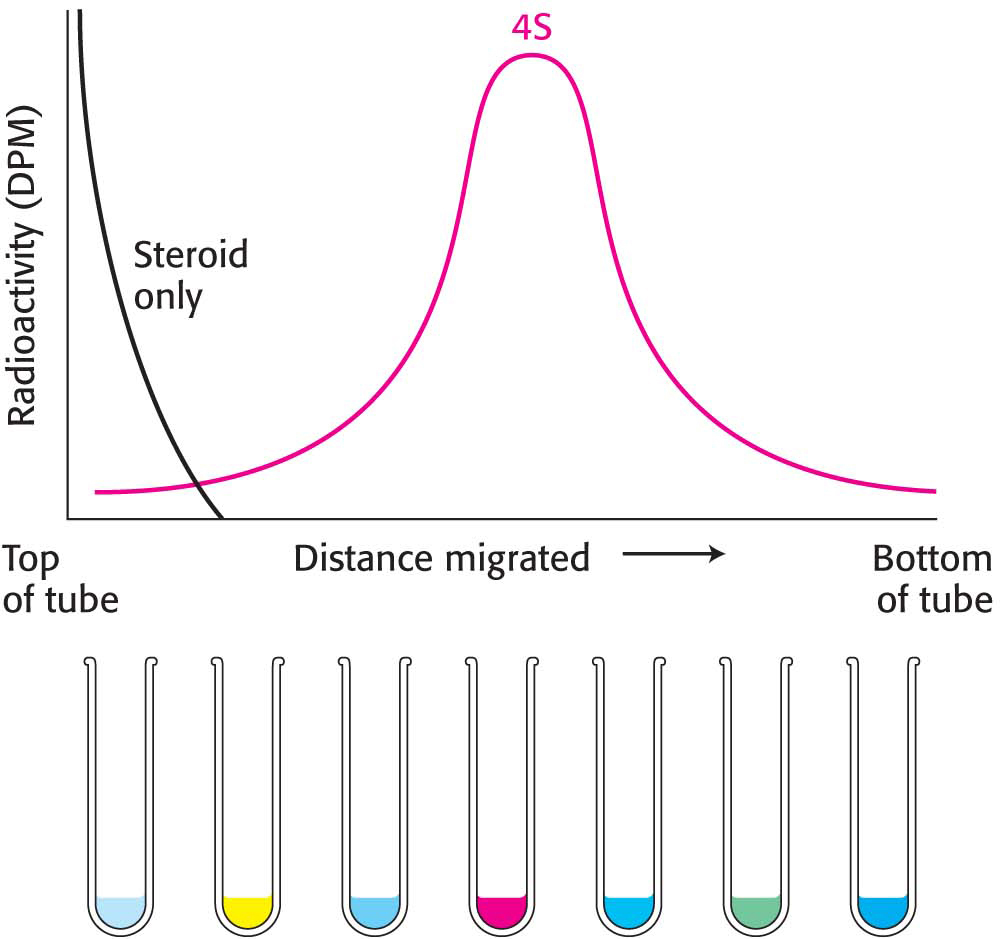
Figure 5.15: Gradient-centrifugation analysis of the estradiol–receptor complex. The receptor protein bound to radioactive estradiol migrates into the gradient on centrifugation. Although some unbound estradiol diffuses into the gradient, the steroid does not migrate to any significant extent. Abbreviation: DpM, disintegrations per minute.
Next, a small volume of a solution containing the mixture of proteins to be separated, which includes the radiolabeled estradiol– receptor complex, is placed on top of the density gradient. When the rotor is spun, proteins move through the gradient and separate according to their sedimentation coefficients. The time and speed of the centrifugation is determined empirically. The separated bands, or zones, of protein can be harvested by making a hole in the bottom of the tube and collecting the solution drop by drop. The drops can be measured for protein content and catalytic activity or another functional property. In regard to the estrogen receptor, the fractions are measured for radioactivity. Figure 5.15 shows the radioactivity of each fraction after centrifugation. Radiolabeled estradiol alone is far too small to move under centrifugal force. Consequently, the radioactivity must be associated with the receptor. A comparison with standards established that the S value of the receptor was 4. However, hundreds of proteins have an S value near 4. Thus, although the radioactivity profile shows only the receptor, there are in fact many other proteins in the same region of the centrifuge tube. Although we can “see” the receptor only, it is still an impure collection of proteins.
Now that we have an assay for the receptor, we need to determine a strategy for purifying the receptor. Because this protein proved difficult to purify with the use of the methods described earlier, we will use immunological techniques. These techniques provide a powerful tool for examining all aspects of biochemical processes and are often the preferred means of protein purification. We will begin by first considering some general immunological properties and then move on to the technique of developing a monoclonal antibody to the estrogen receptor.
Antibodies to Specific Proteins Can Be Generated
Immunological techniques begin with the generation of antibodies to a particular protein. An antibody (also called an immunoglobulin, Ig; Figure 5.16) is itself a protein; it is synthesized by an animal in response to the presence of a foreign substance, called an antigen. Antibodies have specific and high affinity for the antigens that elicited their synthesis. The binding of antibody and antigen is a step in the immune response that protects the animal from infection. Proteins, polysaccharides, and nucleic acids can be effective antigens. When a protein is the antigen, an antibody recognizes a specific group or cluster of amino acids on the target molecule called an antigenic determinant or epitope (Figure 5.17). Animals have a very large repertoire of antibody-producing cells, each synthesizing an antibody of a single specificity. The binding of antigen to antibody stimulates the proliferation of the small number of cells that had already been forming an antibody capable of recognizing the antigen.
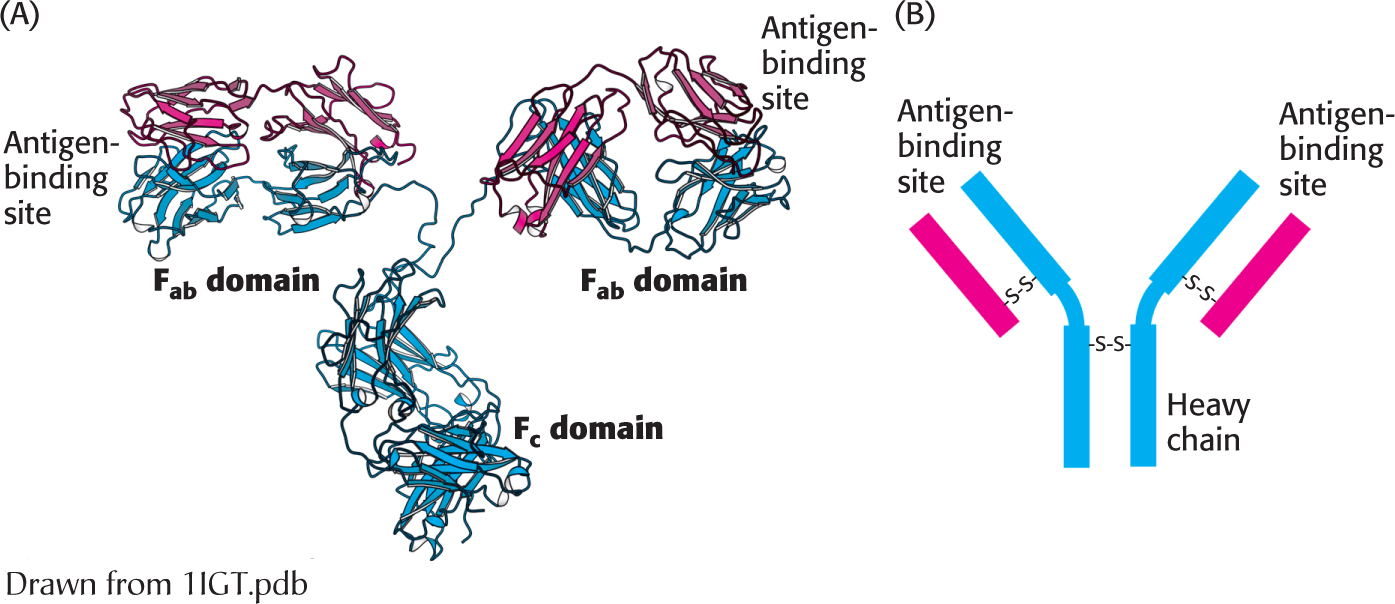
Figure 5.16:  Antibody structure. (A) Immunoglobulin G (IgG) consists of four chains, two heavy chains (blue) and two light chains (red), linked by disulfide bonds. The heavy and light chains come together to form Fab domains, which have the antigen-binding sites at the ends. The two heavy chains form the Fc domain. Notice that the Fab domains are linked to the Fc domain by flexible linkers. (B) A more schematic representation of an IgG molecule.
Antibody structure. (A) Immunoglobulin G (IgG) consists of four chains, two heavy chains (blue) and two light chains (red), linked by disulfide bonds. The heavy and light chains come together to form Fab domains, which have the antigen-binding sites at the ends. The two heavy chains form the Fc domain. Notice that the Fab domains are linked to the Fc domain by flexible linkers. (B) A more schematic representation of an IgG molecule.
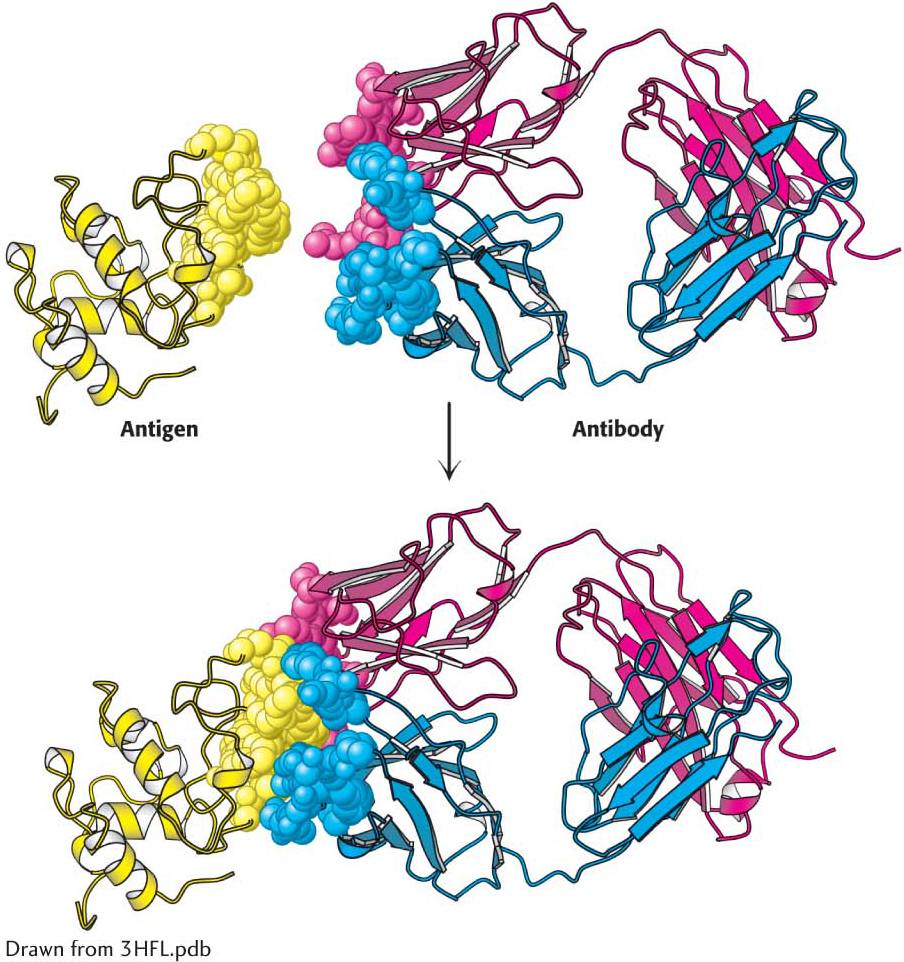
Figure 5.17: 
 Antigen–antibody interactions. A protein antigen—in this case, lysozyme—binds to the end of an Fab domain of an antibody. Notice that the end of the antibody and the antigen have complementary shapes, allowing a large amount of surface to be buried on binding.
Antigen–antibody interactions. A protein antigen—in this case, lysozyme—binds to the end of an Fab domain of an antibody. Notice that the end of the antibody and the antigen have complementary shapes, allowing a large amount of surface to be buried on binding.
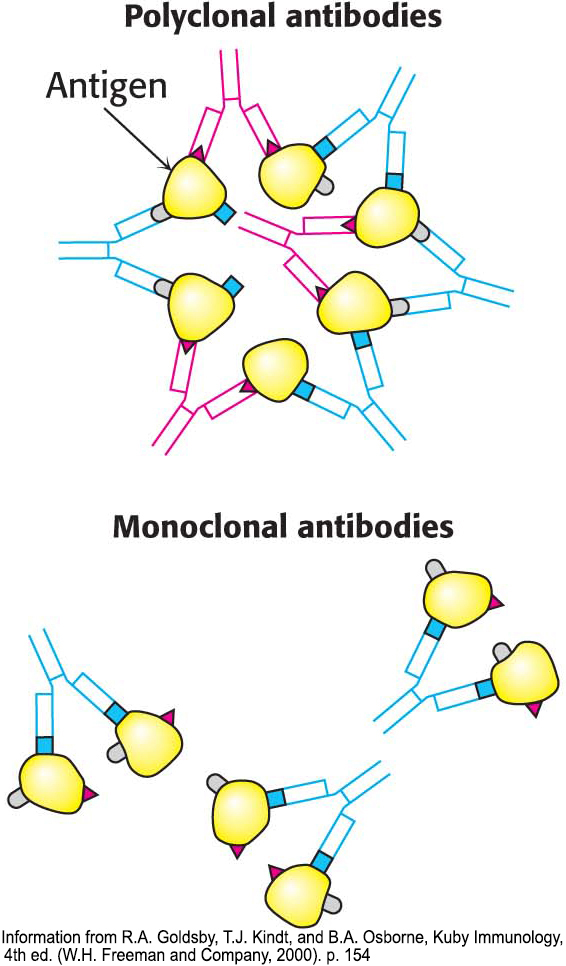
Figure 5.18: Polyclonal and monoclonal antibodies. Most antigens have several epitopes. Polyclonal antibodies are heterogeneous mixtures of antibodies, each specific for one of the various epitopes on an antigen. Monoclonal antibodies are all identical, produced by clones of a single antibody-producing cell. They recognize one specific epitope.
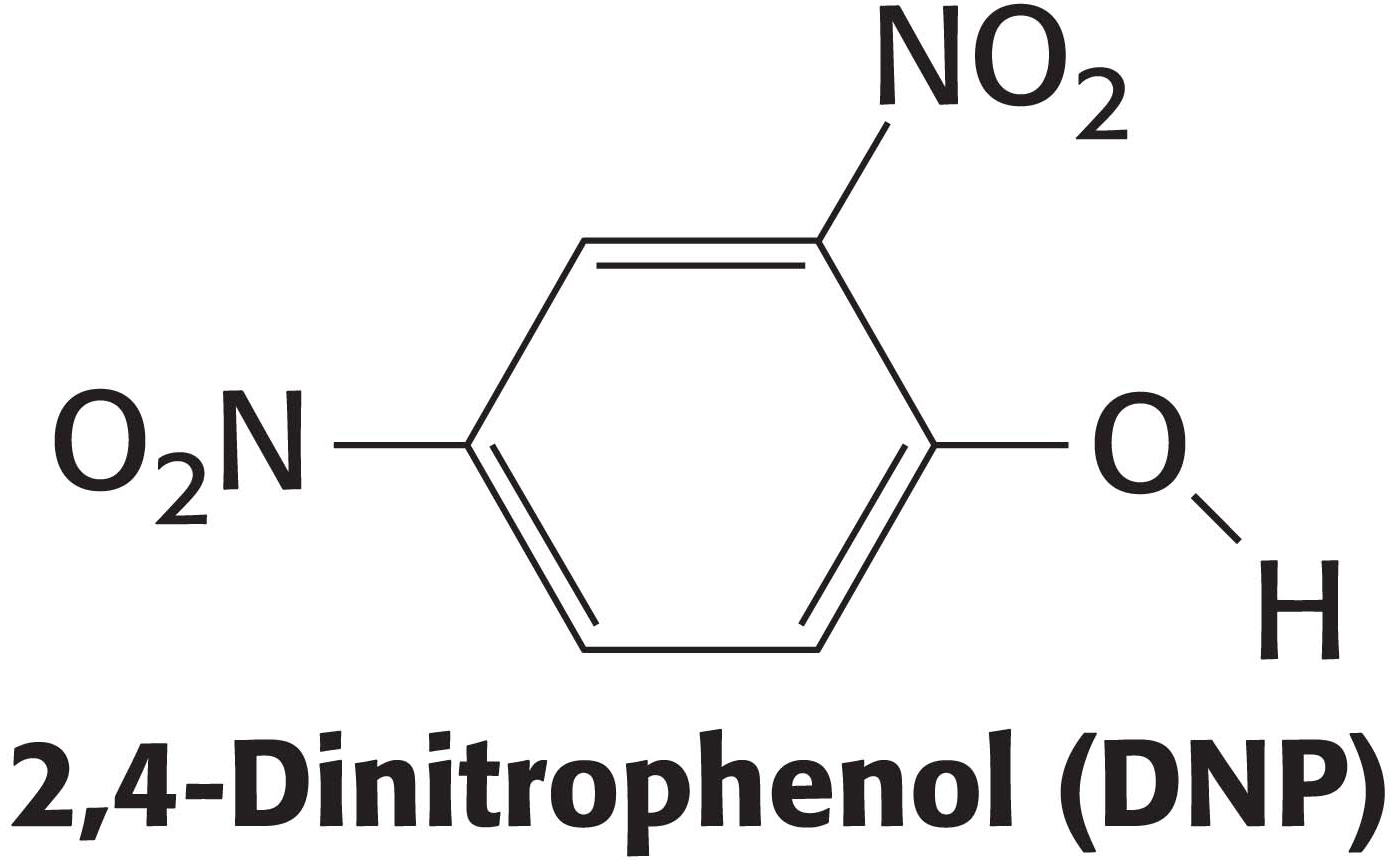
Immunological techniques depend on the ability to generate antibodies to a specific antigen. To obtain antibodies that recognize a particular protein, a biochemist injects the protein into a rabbit. The injected protein stimulates the reproduction of cells producing antibodies that recognize it. Blood is drawn from the immunized rabbit several weeks later and centrifuged to separate blood cells from the supernatant, which is called the serum (blood from which the cells have been removed). The serum, called an antiserum, contains antibodies to all antigens to which the rabbit has been exposed. Only some of them will be antibodies to the injected protein. Moreover, in many cases, many different types of antibody can bind to a single antigen. For instance, consider what occurs when 2,4-dinitrophenol (DNP) is used as an antigen to generate antibodies. Analyses of anti-DNP antibodies revealed a wide range of binding affinities: the dissociation constants ranged from about 0.1 nM to 1 mM. Correspondingly, a large number of bands were evident when anti-DNP antibody was subjected to isoelectric focusing. These results indicate that animals produce many different antibodies, each recognizing a different surface feature of the same antigen. The antibodies are heterogeneous, or polyclonal (Figure 5.18). The heterogeneity of polyclonal antibodies can be advantageous for certain applications, such as the detection of a protein of low abundance, because each protein molecule can be bound by more than one antibody at multiple distinct antigenic sites.
Monoclonal Antibodies with Virtually Any Desired Specificity Can Be Readily Prepared
The discovery of a means of producing monoclonal antibodies of virtually any desired specificity was a major breakthrough that intensified the power of immunological approaches. Just like working with impure proteins, working with an impure mixture of antibodies makes it difficult to interpret data. Rather than separating the target antibody from the mixture, as we might separate a target protein from a mixture of molecules, we will find it more effective to isolate a group of identical cells producing a single kind of antibody for our experiment. The problem is that antibody-producing cells die in a short time in vitro, leaving us little product with which to work.
Immortal cell lines that produce monoclonal antibodies do exist. These cell lines are derived from a type of cancer, multiple myeloma, a malignant disorder of antibody-producing cells. In this cancer, a single transformed plasma cell divides uncontrollably, generating a very large number of cells of a single kind. Such a group of cells is a clone because the cells are descended from the same cell and have identical properties. The identical cells of the myeloma secrete large amounts of normal antibodies of a single kind generation after generation. However, although these antibodies were useful for elucidating antibody structure, nothing is known about their specificity, making them useless for the immunological methods described in the next pages.
César Milstein and Georges Köhler discovered that large amounts of a homogeneous antibody of nearly any desired specificity can be obtained by fusing a short-lived antibody-producing cell with an immortal myeloma cell. An antigen is injected into a mouse, and its spleen, an antibody-producing tissue, is removed several weeks later (Figure 5.19). A mixture of plasma cells from the spleen is fused in vitro with myeloma cells. Each of the resulting hybrid cells, called hybridoma cells, indefinitely produces the identical antibody specified by the parent cell from the spleen. Hybridoma cells will be produced for all of the proteins that were injected into the mouse. The cells can then be screened, by using some sort of specific assay for the antigen–antibody interaction, to determine which ones produce antibodies having the desired specificity. This process is repeated until a pure cell line, a clone producing a single antibody, is isolated.
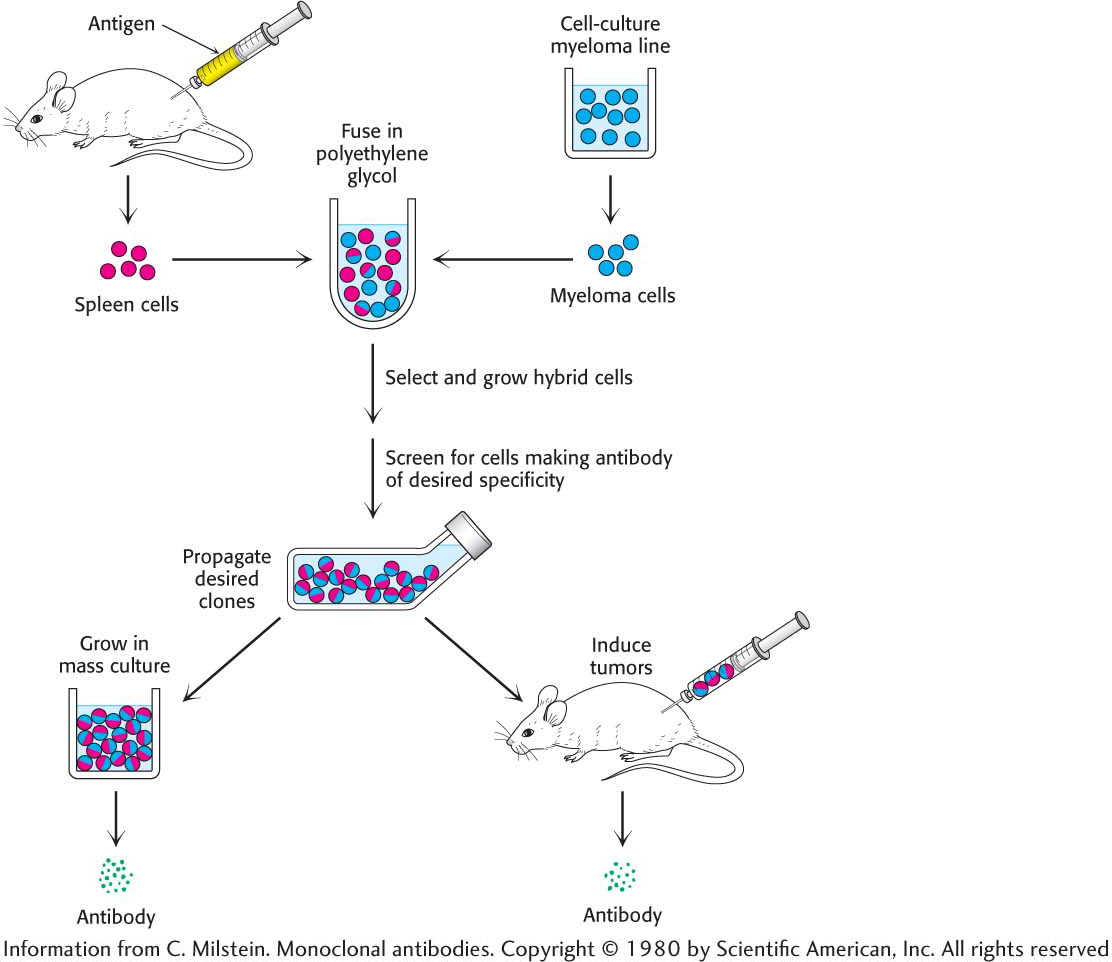
Figure 5.19: The preparation of monoclonal antibodies. Hybridoma cells are formed by the fusion of antibody-producing cells and myeloma cells. The hybrid cells are allowed to proliferate by growing them in selective medium. They are then screened to determine which ones produce antibody of the desired specificity.
DID YOU KNOW?
Clinical laboratories use monoclonal antibodies in many assays. For example, the detection in the blood of enzymes that are normally localized in the heart points to a myocardial infarction (heart attack). Blood transfusions have been made safer by antibody screening of donor blood for viruses that cause AIDS, hepatitis, and other infectious diseases. Monoclonal antibodies also find uses as therapeutic agents. Trastuzumab (herceptin), for example, is a monoclonal antibody useful for treating some forms of breast cancer.
Our goal now is to generate a monoclonal antibody to the estrogen receptor and then to use the antibody to isolate the receptor. To do so, we inject a small amount of cytosol from the rat uterus and generate hybridoma cells as just described. We now have a population of antibodies, some of which are specific for the estrogen receptor. How can we detect estrogen-receptor-specific antibodies? We will use the technique of gradient centrifugation once again. Our reasoning is as follows: if we mix the antibody preparation with the cytosol containing the radiolabeled estradiol–receptor complex, an antibody will bind to the estrogen receptor. This binding should alter the sedimentation profile obtained when we centrifuged the radiolabeled estradiol–receptor complex. Indeed, it is the case. When estradiol–receptor complex is incubated with antibodies, the complex of radioactive steroid and estrogen receptor undergoes sedimentation at 9S in a sucrose gradient, in association with the antibody (Figure 5.20). The population of antibody-producing cells is screened for those that are generating antibodies that bind the estradiol–receptor complex. After a certain number of screens, we will have the pure cell line—a monoclonal cell line—that is producing only one antibody, called a monoclonal antibody, to the estrogen receptor.
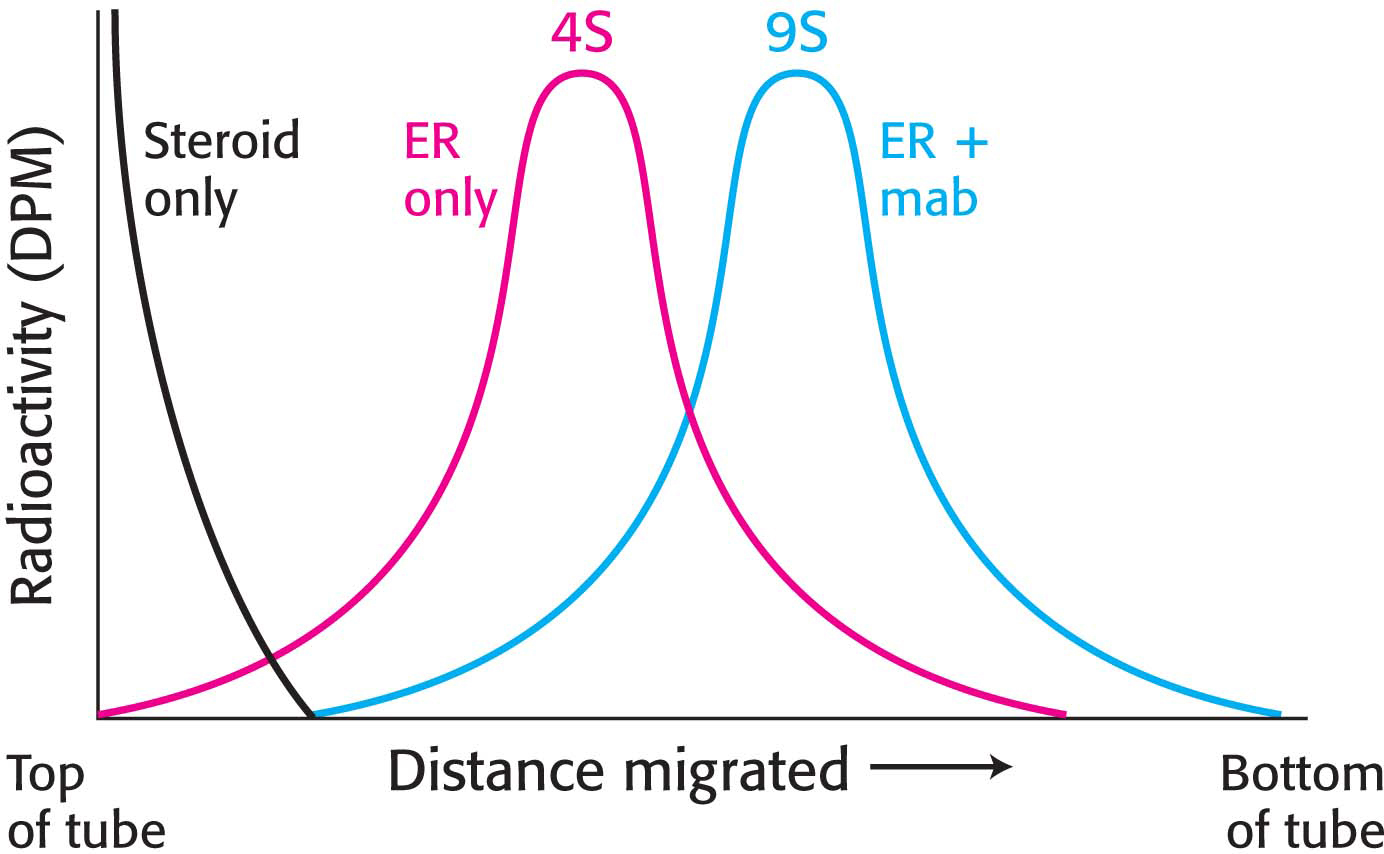
Figure 5.20: Alteration of the sedimentation profile when antibody binds to the receptor protein. The estradiol–receptor–antibody complex migrates farther into the centrifuge tube because it is larger than the estradiol–receptor complex alone. Abbreviations: DPM, disintegrations per minute; ER, estradiol–receptor complex; mab, monoclonal antibody.
The Estrogen Receptor Can Be Purified by ImmunoprecipitationHow can a pure monoclonal antibody to the estrogen receptor be used to isolate pure estrogen receptors from the morass of proteins in the cell cytosol? In essence, we can fish for the estrogen receptor by using these monoclonal antibodies as bait. The monoclonal antibody can be covalently linked to insoluble beads made of polyacrylamide or polysaccharides. The antibody-bound beads are added to a small amount of cytosol and then gently mixed for several hours (Figure 5.21). During this incubation, only the estrogen receptor will bind to the antibody. The mixture is then centrifuged and the beads, along with the attached antibody–antigen complex, will form a pellet at the bottom of the centrifuge tube. The supernatant, which contains all of the non-estrogen-receptor proteins, is discarded. The pellet is then resuspended in buffer and recentrifuged so as to wash away proteins that happened to have been trapped among the beads during the centrifugation. Finally, the addition of a protein denaturant to the mixture causes the estrogen receptor to detach from the antibody. The mixture is recentrifuged, but this time the estrogen receptor remains in the supernatant. The supernatant is collected and used as a source of receptor. This technique, called immunoprecipitation, is similar to affinity chromatography discussed earlier, except here the material bound to the matrix—the antibody—has high affinity for the proteins moving over the matrix.
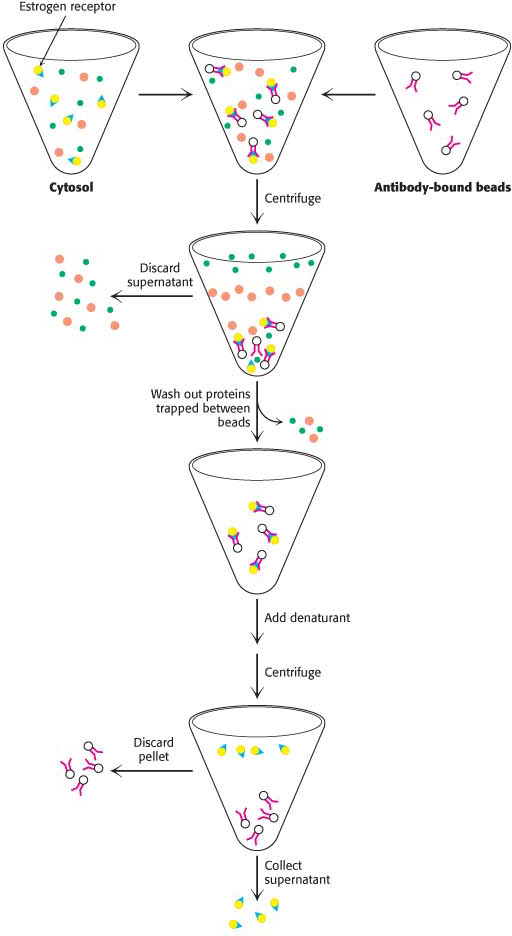
Figure 5.21: Purification by immunoprecipitation. Monoclonal antibody to the estrogen receptor is added to a preparation of cytosol containing the receptor. The antibody is attached to an insoluble bead. The mixture is then gently stirred for several hours to allow the interaction of the receptor and antibody. The mixture is centrifuged and the supernatant is discarded. The pellet is resuspended in buffer to wash out any trapped cytosolic components. The pellet is again collected and the supernatant discarded. The antibody–receptor complex is treated with a denaturant. On centrifugation, the supernatant contains pure estradiol receptor.
Let us take a moment to admire what we have accomplished. We used an impure mixture of proteins containing the estrogen receptor to generate a heterogeneous mixture of antibody-producing cells, a small fraction of which are producing antibodies to the estrogen receptor. Then, we took advantage of two highly specific biochemical interactions—between the receptor and estradiol, and between the receptor and its antibody—to purify, first, a monoclonal antibody and, then, the receptor itself.
Proteins Can Be Detected and Quantified with the Use of an Enzyme-Linked Immunosorbent Assay
Antibodies not only aid in the purification of proteins, but also can be used as reagents to quantify the amount of a protein or other antigen. The technique is the enzyme-linked immunosorbent assay (ELISA). This method makes use of an enzyme that reacts with a colorless substrate to produce a colored product. For instance, peroxidase reacts with hydrogen peroxide and a reduced chromogenic substrate to yield an oxidized colored product. The enzyme is covalently linked to a specific antibody that recognizes a target antigen. If the antigen is present, the antibody–enzyme complex will bind to it; on the addition of the substrate, the enzyme will catalyze the reaction, generating the colored product. Thus, the presence of the colored product indicates the presence of the antigen. If no antigen is present, the enzyme-linked antibody cannot bind to anything and will be washed out of the reaction tube; no color will be generated when the substrate is added. ELISA is rapid and convenient, and can detect less than a nanogram (10−9 g) of a protein. ELISA can be performed with either polyclonal or monoclonal antibodies, but the use of monoclonal antibodies yields more reliable results.
We will consider two among the several types of ELISA. The indirect ELISA is used to detect the presence of antibody and is the basis of the test for HIV infection. The HIV test detects the presence of antibodies that recognize viral core proteins, the antigen. Purified viral core proteins are adsorbed to the bottom of a well. Antibodies from the person being tested are then added to the coated well. Only someone infected with HIV will have antibodies that bind to the antigen. Finally, enzyme-linked antibodies to human antibodies (e.g., enzyme-linked goat antibodies whose antigens are human antibodies) are allowed to react in the well, and unbound antibodies are removed by washing. Substrate is then applied. An enzyme reaction suggests that the enzyme-linked antibodies were bound to human antibodies, which in turn implies that the patient has antibodies to the viral antigen (Figure 5.22).
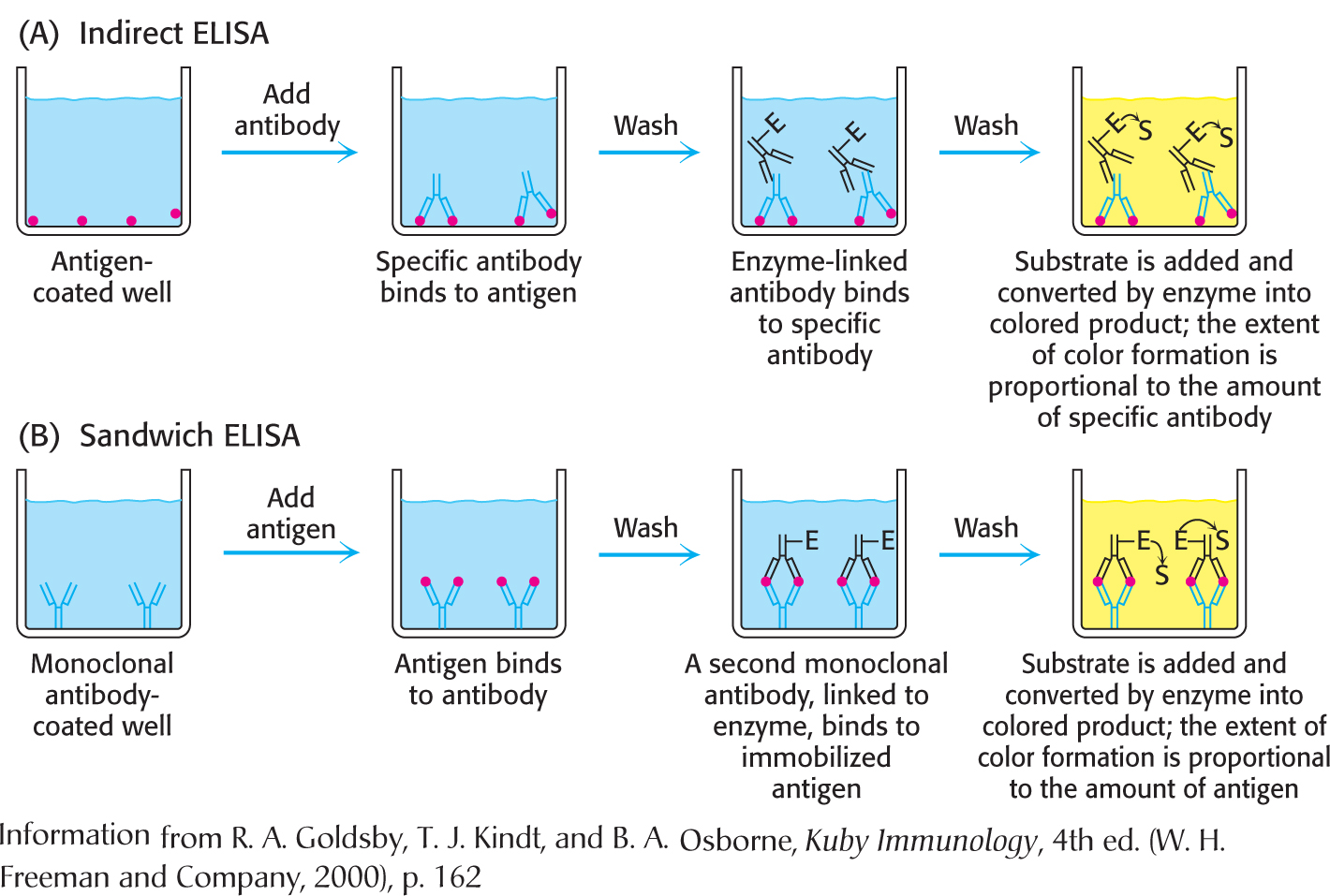
Figure 5.22: Indirect ELISA and sandwich ELISA. (a) In indirect ELISA, the production of color indicates the amount of an antibody to a specific antigen. (B) In sandwich ELISA, the production of color indicates the quantity of antigen.
The sandwich ELISA is used to detect antigen rather than antibody. Antibody to a particular antigen is first adsorbed to the bottom of a well. This antibody is called the capture antibody. Next, blood or urine containing the antigen is added to the well and binds to the antibody. Finally, a second, different antibody to the antigen, the detection antibody, is added. This antibody is enzyme linked and is processed as described for indirect ELISA. In this case, the extent of color formation is directly proportional to the amount of antigen present. Consequently, it permits the measurement of small quantities of antigen (Figure 5.22). The sandwich ELISA is the basis of home pregnancy tests.
Western Blotting Permits the Detection of Proteins Separated by Gel Electrophoresis
!quickquiz! QUICK QUIZ 3
What is the biochemical basis for the power of immunological techniques?
ELISA is a powerful technique for screening large numbers of samples for the presence of particular antibodies or antigens. Another immunoassay technique called western blotting or immunoblotting allows the detection of very small quantities of a particular protein in a cell or in body fluid (Figure 5.23). Western blotting also allows the determination of the size of the target protein. A key step in western blotting is to subject the sample under investigation to electrophoresis on an SDS– polyacrylamide gel to separate the individual proteins. How can we detect the presence of the protein of interest? In principle, we could soak the gel in a solution of antibody and detect the protein. However, antibodies are too large to penetrate the gel. To make the proteins accessible to the antibody, the resolved proteins on the gel are transferred to the surface of a polymer sheet (blotted) by an electrical current. An antibody that is specific for the protein of interest is added to the sheet and reacts with the antigen. The antibody–antigen complex on the sheet can then be detected by rinsing the sheet with a fluorescently labeled antibody specific for the first (e.g., goat antibody that recognizes mouse antibody). The fluorescently labeled second antibody reveals the presence of the first antibody. Alternatively, an enzyme on the second antibody generates a colored product, as in the ELISA method. Western blotting makes it possible to find a protein in a complex mixture, the proverbial needle in a haystack. It is the basis for the test for infection by hepatitis C, where it is used to detect a core protein of the virus. This technique is also very useful in monitoring protein purification and in the cloning of genes.
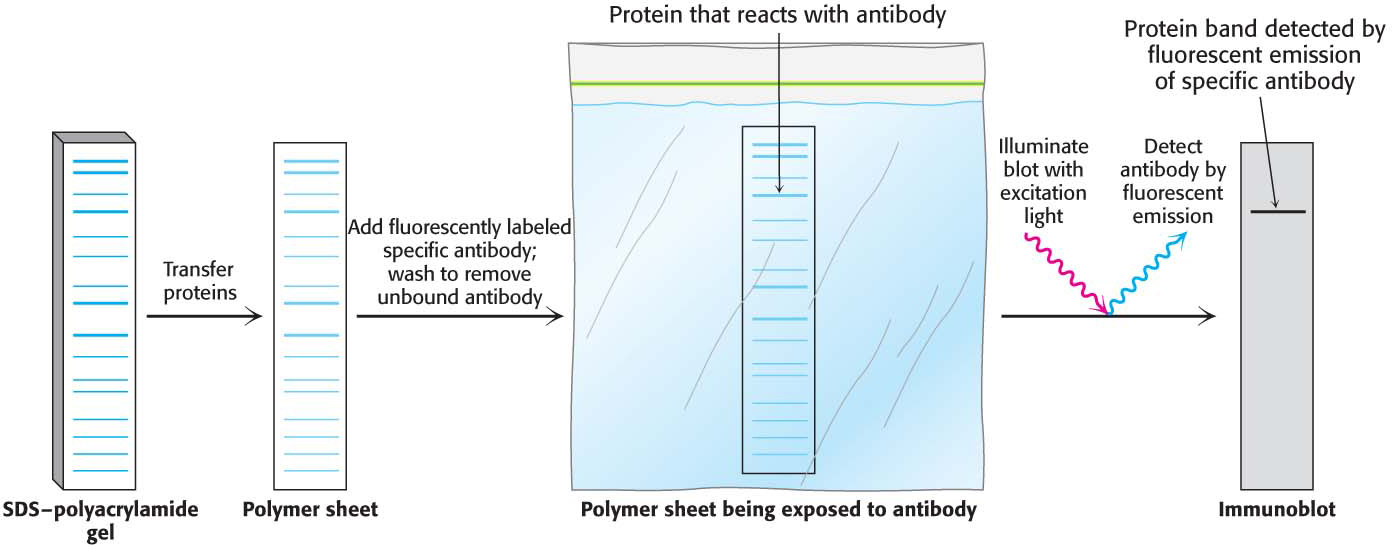
Figure 5.23: Western blotting. Proteins on an SDS–polyacrylamide gel are transferred to a polymer sheet and stained with fluorescent antibody. The fluorescent antibody is excited by light, and the band corresponding to the protein to which the antibody binds is visualized with an appropriate detector.

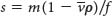
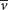 is the partial specific volume (the reciprocal of the particle density), ρ is the density of the medium, and f is the frictional coefficient (a measure of the shape of the particle). The
is the partial specific volume (the reciprocal of the particle density), ρ is the density of the medium, and f is the frictional coefficient (a measure of the shape of the particle). The 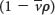 term is the buoyant force exerted by the liquid medium.
term is the buoyant force exerted by the liquid medium.
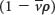 is smaller for the denser particle.
is smaller for the denser particle.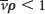 , float when
, float when 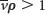 , and do not move when
, and do not move when 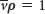 .
.


 Antibody structure. (A) Immunoglobulin G (IgG) consists of four chains, two heavy chains (blue) and two light chains (red), linked by disulfide bonds. The heavy and light chains come together to form Fab domains, which have the antigen-
Antibody structure. (A) Immunoglobulin G (IgG) consists of four chains, two heavy chains (blue) and two light chains (red), linked by disulfide bonds. The heavy and light chains come together to form Fab domains, which have the antigen-

 Antigen–
Antigen–