8.3 Chymotrypsin Illustrates Basic Principles of Catalysis and Inhibition
A detailed examination of the mechanism of action of the protein-degrading enzyme chymotrypsin will illustrate some of the basic principles of catalysis. It will also be useful as a case study for showing how enzyme mechanisms can be investigated, including the use of kinetics and enzyme inhibitors.
Protein turnover is an important process in living systems. After they are no longer needed in the cell, proteins must be degraded so that their constituent amino acids can be recycled for the synthesis of new proteins. Additionally, proteins ingested in the diet must be broken down into small peptides and amino acids for absorption in the intestine. Protein breakdown is catalyzed by a large class of enzymes called proteolytic enzymes or proteases. These enzymes cleave proteins by a hydrolysis reaction—the addition of a molecule of water to a peptide bond.
One such proteolytic enzyme is chymotrypsin, which is secreted by the pancreas in response to a meal. Chymotrypsin cleaves peptide bonds selectively on the carboxyl-terminal side of the large hydrophobic amino acids such as tryptophan, tyrosine, phenylalanine, and methionine (Figure 8.20).
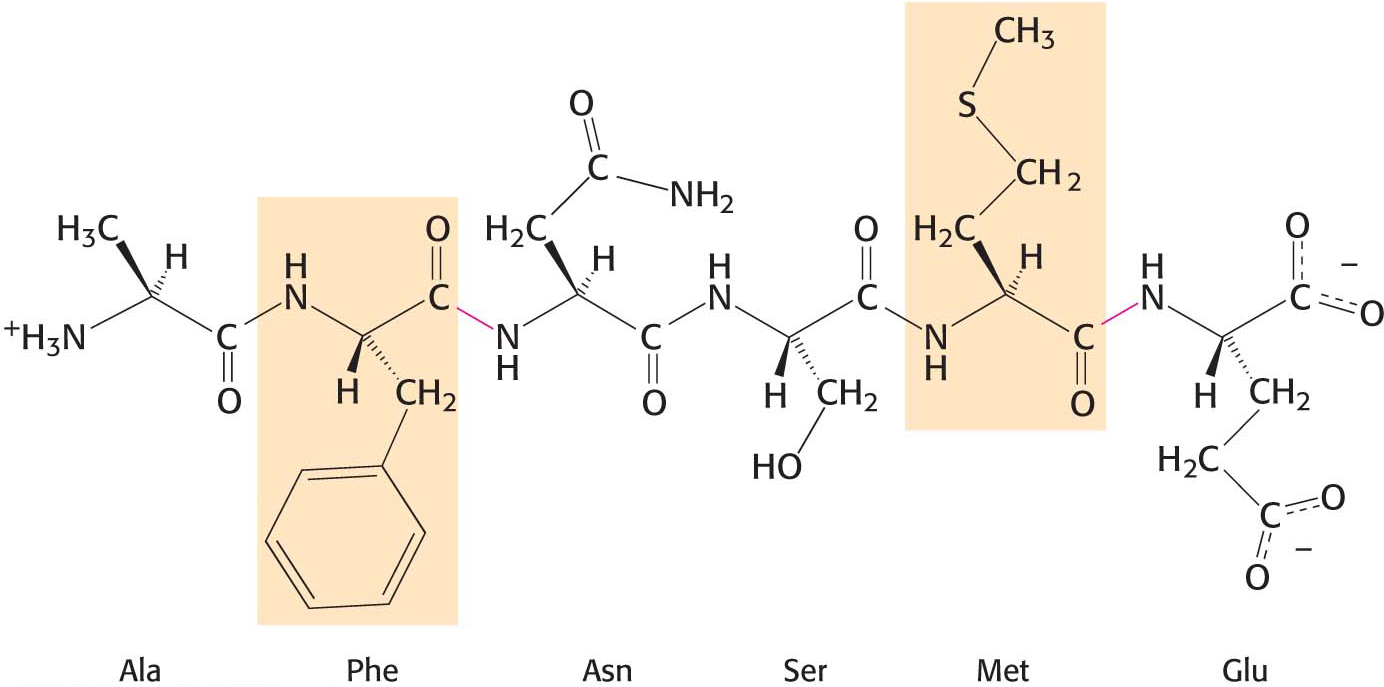
Figure 8.20: The specificity of chymotrypsin. Chymotrypsin cleaves proteins on the carboxyl side of aromatic or large hydrophobic amino acids (shaded orange). The red bonds indicate where chymotrypsin most likely acts.
Serine 195 Is Required for Chymotrypsin Activity
Chymotrypsin is a good example of the use of covalent modification as a catalytic strategy. The enzyme employs a powerful nucleophile to attack the unreactive carbonyl group of the substrate. This nucleophile becomes covalently attached to the substrate briefly in the course of catalysis.
What is the nucleophile that chymotrypsin employs to attack the substrate carbonyl group? A clue came from the fact that chymotrypsin contains an extraordinarily reactive serine residue. Treatment with organofluorophosphates that modify serine residues, such as DIPF, was found to inactivate the enzyme irreversibly (Figure 8.13). Despite the fact that the enzyme possesses 28 serine residues, only one of them, serine 195, was modified, resulting in a total loss of enzyme activity. The use of the group-specific reagent DIPF alerted researchers to the importance of one particular serine residue in catalysis.
Chymotrypsin Action Proceeds in Two Steps Linked by a Covalently Bound Intermediate
A study of the enzyme’s kinetics suggested a role for serine 195. The kinetics of enzyme action are often easily monitored by having the enzyme act on a substrate analog that forms a colored product. For chymotrypsin, such a chromogenic substrate is N-acetyl-l-phenylalanine p-nitrophenyl ester. One of the products formed by chymotrypsin’s cleavage of this substrate is p-nitrophenolate, which has a yellow color (Figure 8.21). Measurements of the absorbance of light revealed the amount of p-nitrophenolate being produced and thus provided a facile means of investigating chymotrypsin activity.
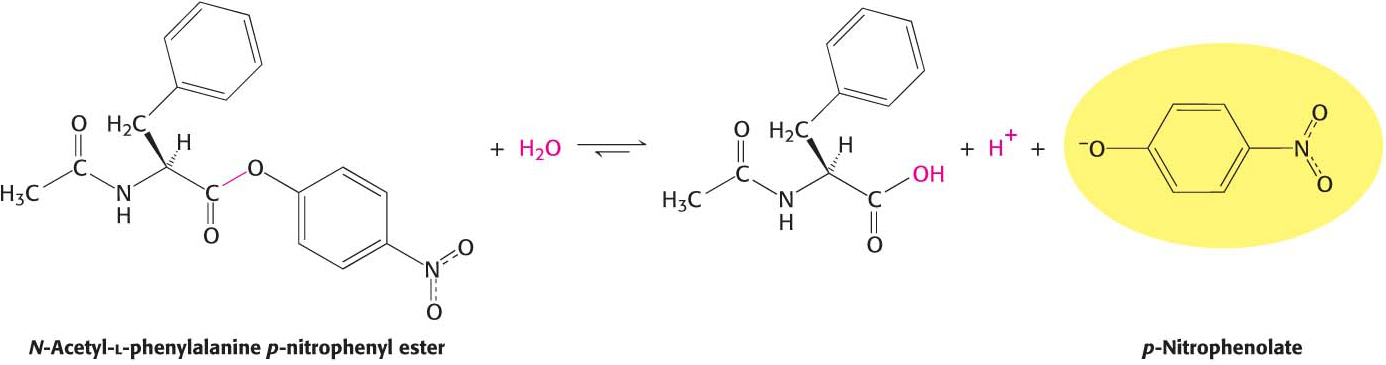
Figure 8.21: A chromogenic substrate. N-Acetyl-l-phenylalanine p-nitrophenyl ester yields a yellow product, p-nitrophenolate, on cleavage by chymotrypsin. p-Nitrophenolate forms by deprotonation of p-nitrophenol at pH 7.
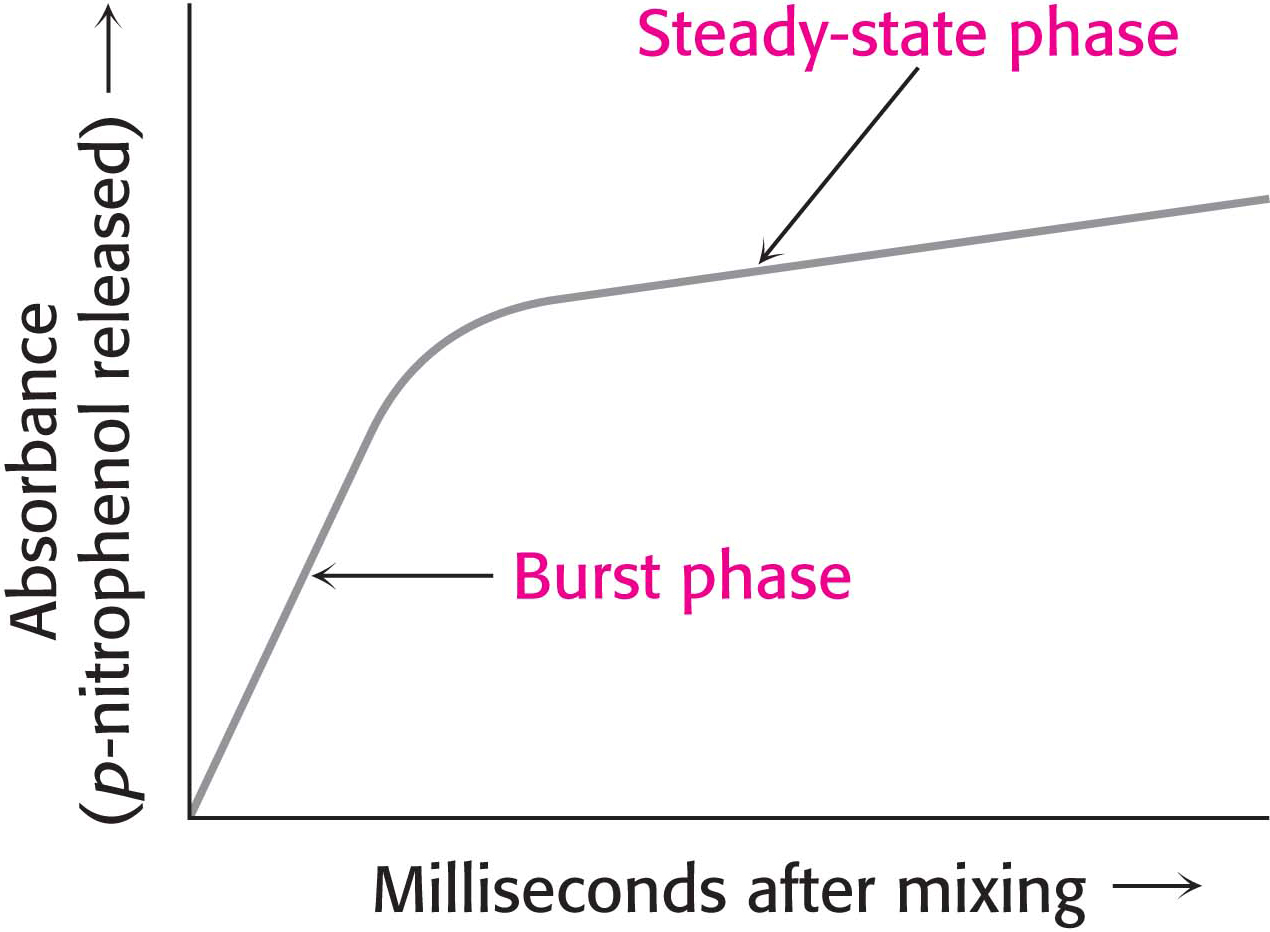
Figure 8.22: Kinetics of chymotrypsin catalysis. Two stages are evident in the cleaving of N-acetyl-l-phenylalanine p-nitrophenyl ester by chymotrypsin: a rapid burst phase (pre-steady state) and a steady-state phase.
DID YOU KNOW?
In a steady-state system, the concentrations of the intermediates stay the same, even though the concentrations of substrate and products are changing. A sink filled with water that has the tap open just enough to match the loss of water down the drain is in a steady state. The level of the water in the sink never changes even though water is constantly flowing from the faucet through the sink and out through the drain.
Under steady-state conditions, the cleavage of the substrate obeys Michaelis–Menten kinetics. More insightful results were obtained by examining product formation within milliseconds of mixing the enzyme and substrate. The reaction between chymotrypsin and N-acetyl-l-phenylalanine p-nitrophenyl ester produced an initial rapid burst of colored product, followed by its slower formation as the reaction reached the steady state (Figure 8.22). These results suggest that hydrolysis proceeds in two steps. The burst is observed because the first step is more rapid than the second step.
The two steps are explained by the formation of a covalently bound enzyme–substrate intermediate (Figure 8.23). First, the acyl group of the substrate becomes covalently attached to serine 195 of the enzyme as p-nitrophenolate is released. The enzyme–acyl-group complex is called the acyl-enzyme intermediate. Second, the acyl-enzyme intermediate is hydrolyzed to release the carboxylic acid component of the substrate and regenerate the free enzyme. Thus, one molecule of p-nitrophenolate is produced rapidly from each enzyme molecule as the acyl-enzyme intermediate is formed. However, it takes longer for the enzyme to be “reset” by the hydrolysis of the acyl-enzyme intermediate, and both steps are required for enzyme turnover.
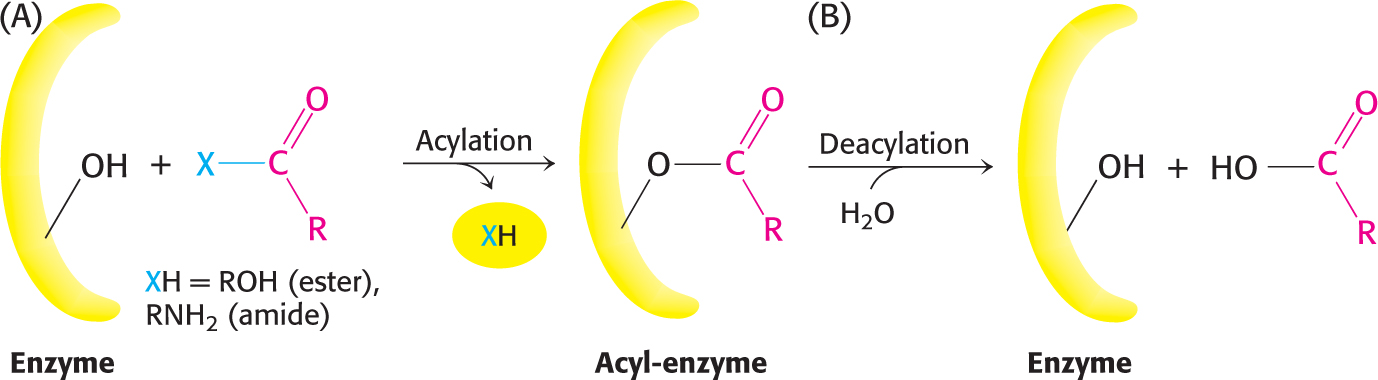
Figure 8.23: Covalent catalysis. Hydrolysis by chymotrypsin takes place in two stages: (A) acylation to form the acyl-enzyme intermediate followed by (B) deacylation to regenerate the free enzyme.
Serine Is Part of a Catalytic Triad That Includes Histidine and Aspartic Acid
Thus far, we have learned that serine 195 and histidine 57 are required for chymotrypsin activity, and the reaction proceeds through a substituted-enzyme intermediate. How can we integrate this information to elucidate the mechanism of chymotrypsin action? The side chain of serine 195 is hydrogen bonded to the imidazole ring of histidine 57. The—NH group of this imidazole ring is, in turn, hydrogen bonded to the carboxylate group of aspartate 102, another key component of the active site. This constellation of residues is referred to as the catalytic triad.
How does this arrangement of residues lead to the high reactivity of serine 195? The histidine residue serves to position the serine side chain and to polarize its hydroxyl group so that it is poised for deprotonation. In the presence of the substrate, histidine 57 accepts the proton from the serine-195 hydroxyl group. In doing so, histidine acts as a general base catalyst. The withdrawal of the proton from the hydroxyl group generates an alkoxide ion, which is a much more powerful nucleophile than a hydroxyl group is. The aspartate residue helps orient the histidine residue and make it a better proton acceptor through hydrogen bonding and electrostatic effects (Figure 8.24).
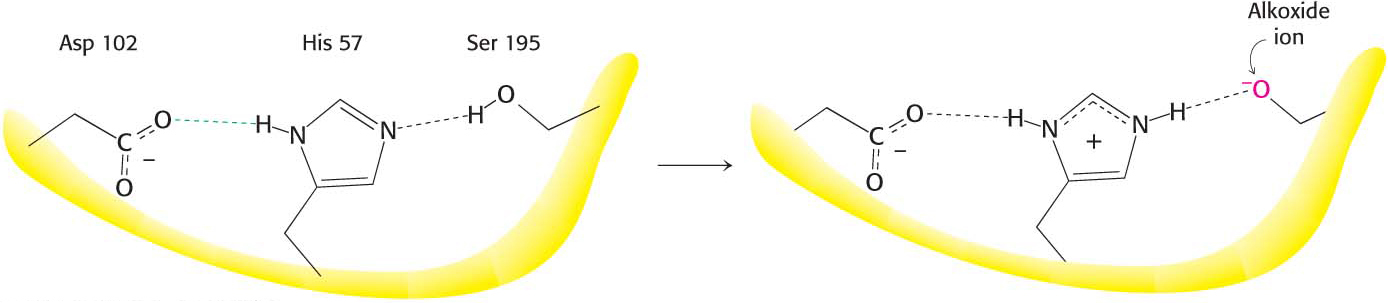
Figure 8.24: The catalytic triad. The catalytic triad, shown on the left, converts serine 195 into a potent nucleophile, as illustrated on the right.
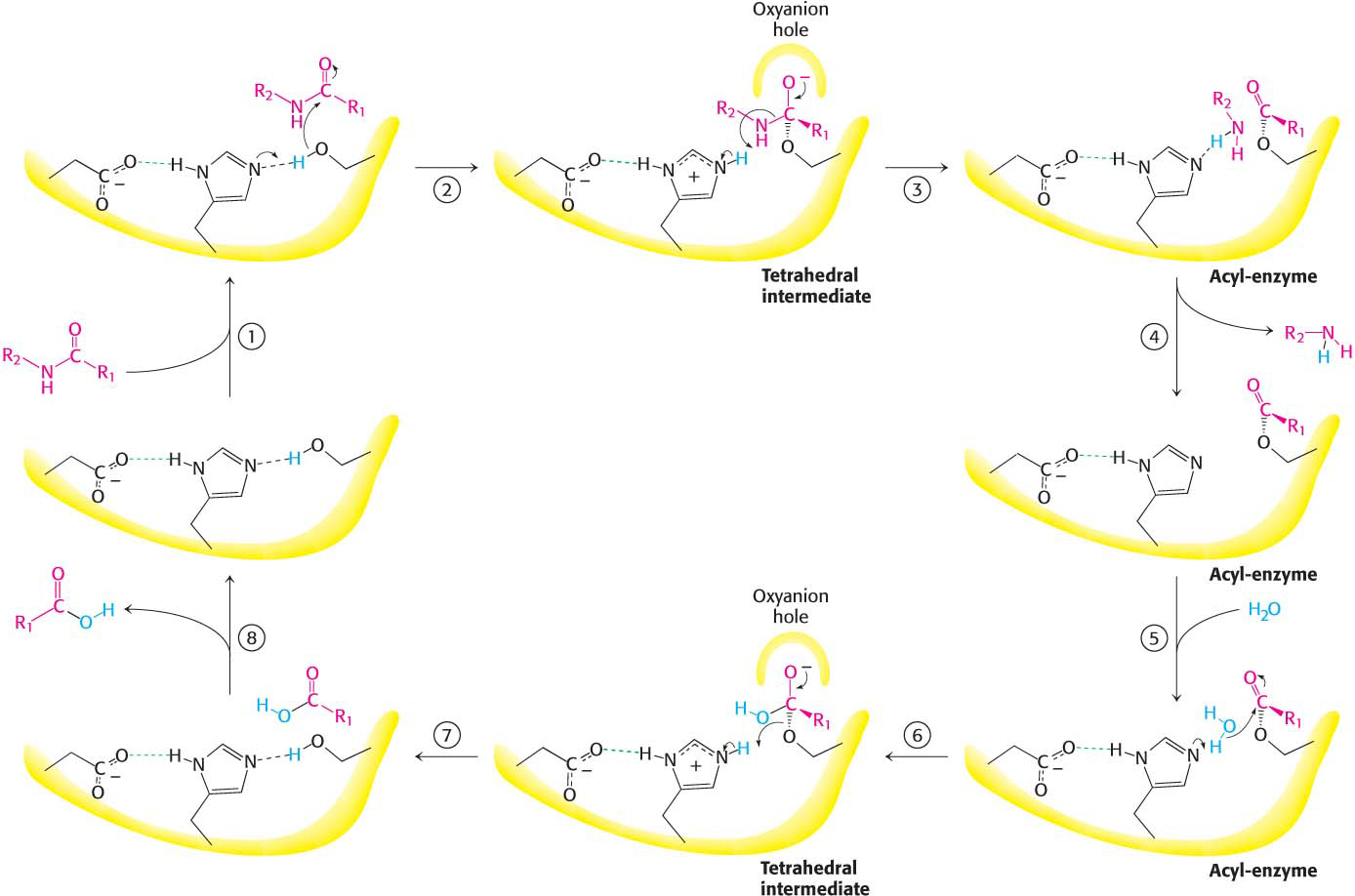
Figure 8.25: Peptide hydrolysis by chymotrypsin. The mechanism of peptide hydrolysis illustrates the principles of covalent and acid–base catalysis. The reaction proceeds in eight steps: (1) substrate binding, (2) serine’s nucleophilic attack on the peptide carbonyl group, (3) collapse of the tetrahedral intermediate, (4) release of the amine component, (5) water binding, (6) water’s nucleophilic attack on the acyl-enzyme intermediate, (7) collapse of the tetrahedral intermediate, and (8) release of the carboxylic acid component. The dashed green lines represent hydrogen bonds.
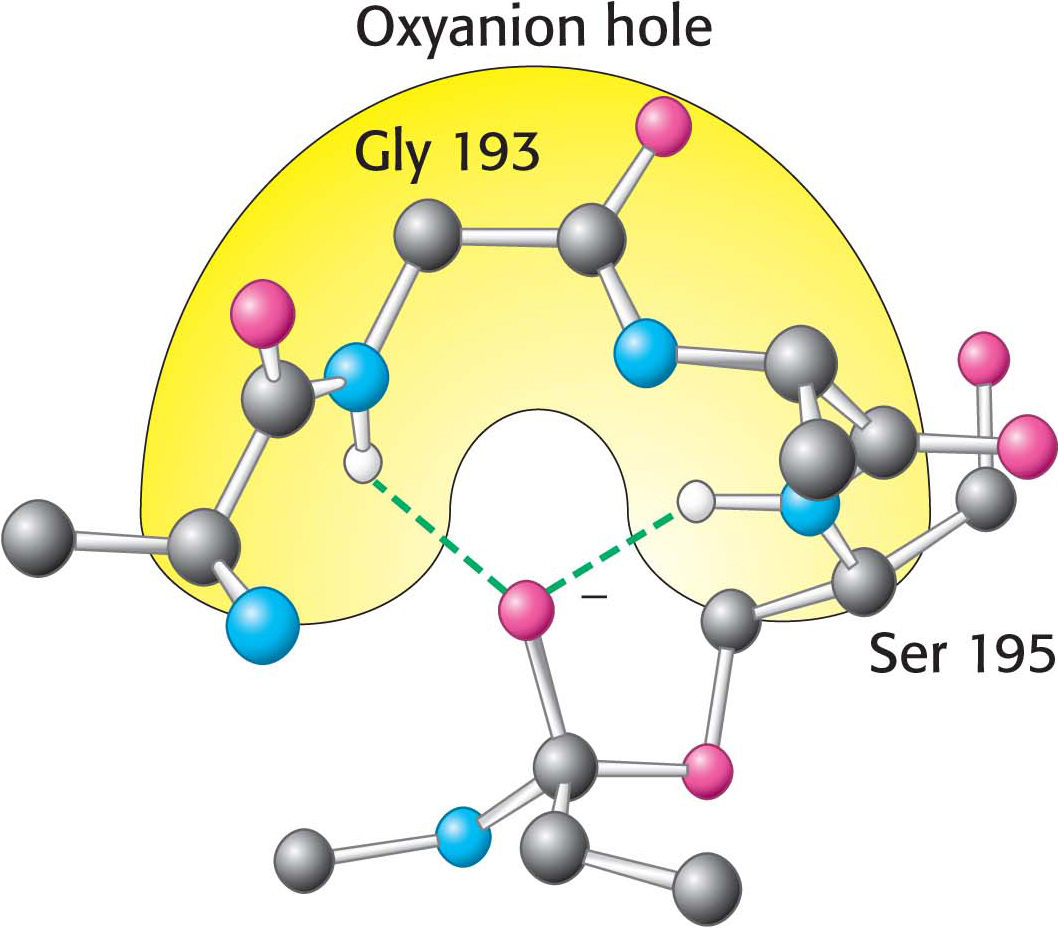
Figure 8.26: The oxyanion hole. The structure stabilizes the tetrahedral intermediate of the chymotrypsin reaction. Notice that hydrogen bonds (shown in green) link peptide NH groups and the negatively charged oxygen atom of the intermediate.
These observations suggest a mechanism for peptide hydrolysis (Figure 8.25). After substrate binding (step 1), the reaction begins with the oxygen atom of the side chain of serine 195 making a nucleophilic attack on the carbonyl carbon atom of the target peptide bond (step 2). There are now four atoms bonded to the carbonyl carbon atom, arranged as a tetrahedron, instead of three atoms in a planar arrangement. The inherently unstable tetrahedral-intermediate form bears a negative charge on the oxygen atom derived from the carbonyl group. This charge is stabilized by a site termed the oxyanion hole, as shown in Figure 8.25. Interactions with NH groups in the oxyanion hole help to stabilize the tetrahedral intermediate (Figure 8.26). These interactions contribute to the binding energy that helps stabilize the transition state that precedes the formation of the tetrahedral intermediate (Figure 8.25). This tetrahedral intermediate collapses to generate the acyl-enzyme (step 3). This step is facilitated by the transfer of the proton from the positively charged histidine residue, now acting as a general acid catalyst, to the amino group of the substrate formed by cleavage of the peptide bond. The amine component is now free to depart from the enzyme (step 4), completing the first stage of the hydrolytic reaction—that is, acylation of the enzyme.
The next stage—deacylation of the enzyme—begins when a water molecule takes the place occupied earlier by the amine component of the substrate (step 5). The ester group of the acyl-enzyme is now hydrolyzed by a process that essentially repeats steps 2 through 4. Again acting as a general base catalyst, histidine 57 draws a proton away from the water molecule. The resulting OH– ion attacks the carbonyl carbon atom of the acyl group, forming a tetrahedral intermediate (step 6). This structure breaks down to form the carboxylic acid product (step 7). Finally, the release of the carboxylic acid product readies the enzyme for another round of catalysis (step 8).
This mechanism accounts for all characteristics of chymotrypsin action except the observed preference for cleaving the peptide bonds just past residues with large, hydrophobic side chains. Examination of the three-dimensional structure of chymotrypsin with substrate analogs and enzyme inhibitors revealed the presence of a deep, relatively hydrophobic pocket, called the S1 pocket, into which the long, uncharged side chains of residues such as phenylalanine and tryptophan can fit. The binding of an appropriate side chain into this pocket positions the adjacent peptide bond into the active site for cleavage (Figure 8.27). The specificity of chymotrypsin depends almost entirely on which amino acid is directly on the amino-terminal side of the peptide bond to be cleaved.
!quickquiz! QUICK QUIZ 2
Using the Cleland representation (Figure 7.6), show the reaction progress of the hydrolysis of the tetrapeptide Ala-Phe-Gly-Ala by chymotrypsin.
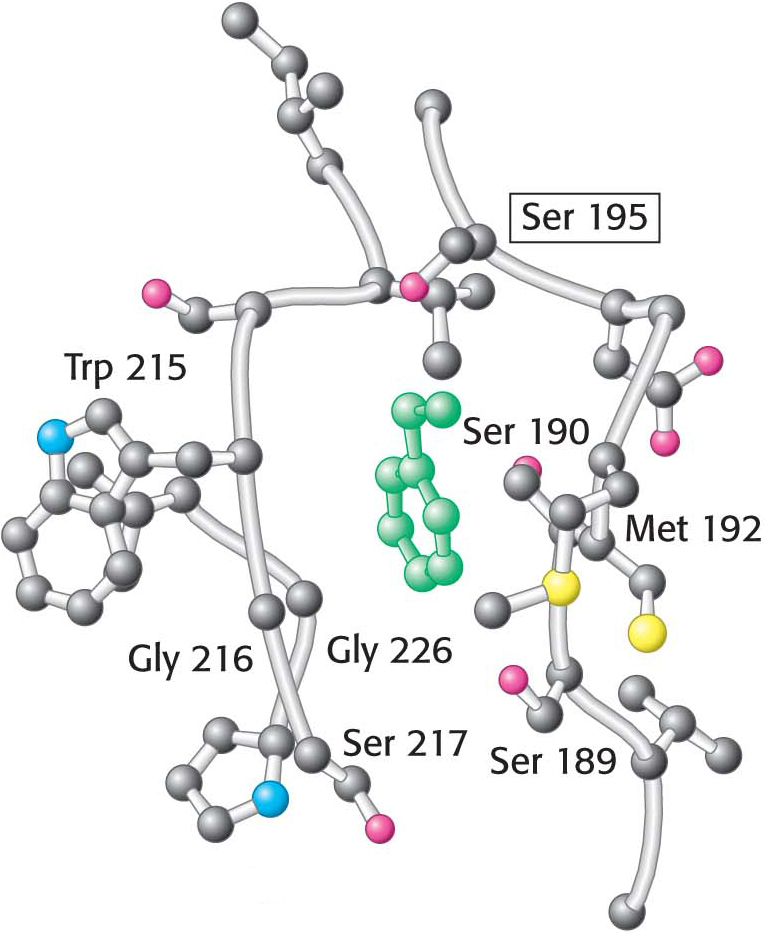
Figure 8.27: The specificity pocket of chymotrypsin. Notice that many hydrophobic groups line the deep specificity pocket. The structure of the pocket favors the binding of residues with long hydrophobic side chains such as phenylalanine (shown in green). Also notice that the active-site serine residue (serine 195) is positioned to cleave the peptide backbone between the residue bound in the pocket and the next residue in the sequence. The key amino acids that constitute the binding site are identified.







