Appendix 3: Chemical Reactions
Electron Shells and Ion Stability
Electrons surround the nucleus of an atom of each element in a unique set of concentric spheres called electron shells. Each shell can hold a certain maximum number of electrons. In the chemical reactions of most elements, only the electrons in the outermost shells interact. In the reaction between sodium (Na) and chlorine (Cl) that forms sodium chloride (NaCl), for example, the sodium atom loses an electron from its outer shell of electrons, and the chlorine atom gains an electron in its outer shell (see Figure 3.4).
Before reacting with chlorine, the sodium atom has one electron in its outer shell. When it loses that electron, its outer shell is eliminated and the next shell inward, which has eight electrons (the maximum that shell can hold), becomes the outer shell. The original chlorine atom had seven electrons in its outer shell, with room for a total of eight. By gaining an electron, it fills its outer shell. Many elements have a strong tendency to acquire a full outer electron shell, some by gaining electrons and some by losing them in the course of a chemical reaction.
Many chemical reactions entail gains and losses of several electrons as two or more elements combine. The element calcium (Ca), for example, becomes a doubly charged cation, Ca2+, as it reacts with two chlorine atoms to form calcium chloride. In the chemical formula for calcium chloride, CaCl2, the presence of two chloride ions is symbolized by the subscript 2. Chemical formulas thus show the relative proportions of atoms or ions in a compound. Common practice is to omit the subscript 1 next to single ions in a formula.
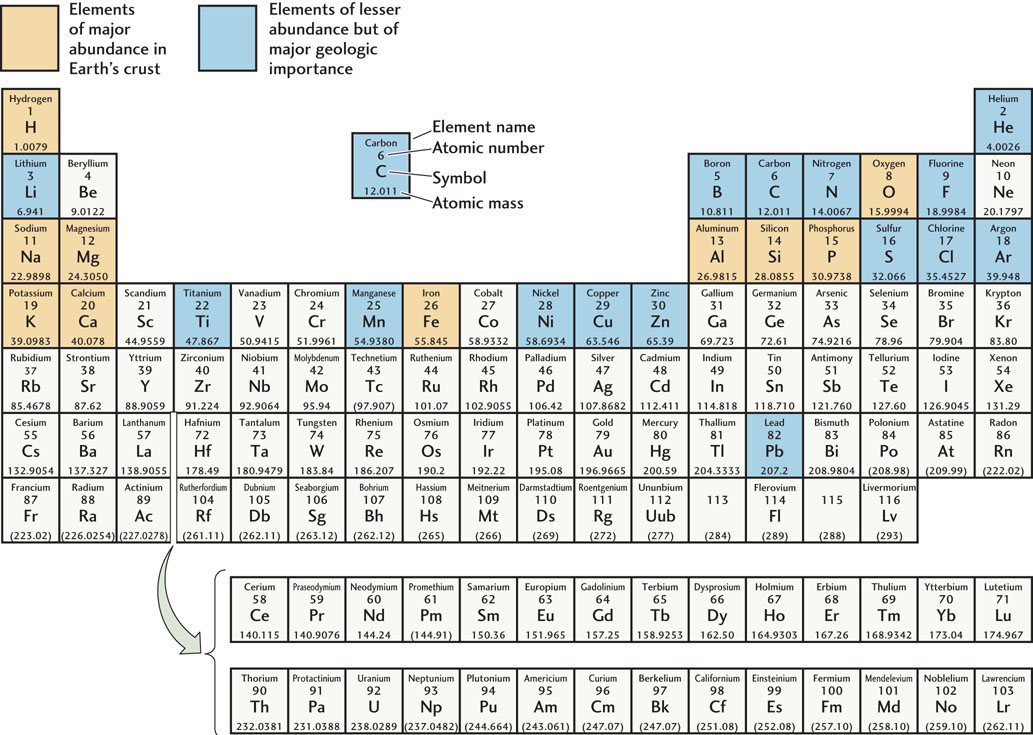
The periodic table organizes the elements (from left to right along a row) in order of atomic number (the number of protons), which also means increasing the numbers of electrons in the outer shell. The third row from the top, for example, starts at the left with sodium (atomic number 11), which has one electron in its outer shell. The next is magnesium (atomic number 12), which has two electrons in its outer shell, followed by aluminum (atomic number 13), with three, and silicon (atomic number 14), with four. Then come phosphorus (atomic number 15), with five; sulfur (atomic number 16), with six; and chlorine (atomic number 17), with seven. The last element in this row is argon (atomic number 18), with eight electrons, the maximum possible, in its outer shell. Each column in the table forms a vertical grouping of elements with similar electron-shell patterns.
AP-4
Elements That Tend to Lose Electrons
The elements in the leftmost column of the table all have a single electron in their outer shells and have a strong tendency to lose that electron in chemical reactions. Of this group, hydrogen (H), sodium (Na), and potassium (K) are found in major abundance at Earth’s surface and in its crust.
The second column from the left includes two more elements that are abundant on Earth: magnesium (Mg) and calcium (Ca). Elements in this column have two electrons in their outer shells and a strong tendency to lose both of them in chemical reactions.
Elements That Tend to Gain Electrons
Toward the right side of the table, the two columns headed by oxygen (O), the most abundant element on Earth, and fluorine (F), a highly reactive toxic gas, contain elements that tend to gain electrons in their outer shells. The elements in the column headed by oxygen have six of the possible eight electrons in their outer shells and tend to gain two electrons. Those in the column headed by fluorine have seven electrons in their outer shells and tend to gain one.
Other Elements
The elements in the columns between those farthest to the left and those farthest to the right have varying tendencies to gain, lose, or share electrons. The column toward the right of the table headed by carbon (C) includes silicon (Si), another of the most abundant elements on Earth. Both silicon and carbon tend to share electrons.
The elements in the last column on the right, headed by helium (He), have full outer shells and thus no tendency either to gain or to lose electrons. As a result, these elements, in contrast with those in other columns, do not react chemically with other elements, except under very special conditions.
AP-5