The Biosphere as a System
Life is everywhere on Earth. The biosphere is that part of our planet that contains all of its living organisms. It includes the plants and animals with which we are most familiar as well as the nearly invisible microorganisms that live in some of the most extreme environments on Earth. These organisms live on Earth’s surface, in its atmosphere and ocean, and within its upper crust, and they interact continuously with all of these environments. Because the biosphere intersects with the lithosphere, hydrosphere, and atmosphere, it can influence or even control basic geologic and climate processes. Geobiology is the study of these interactions between the biosphere and Earth’s physical environment.
The biosphere is a system of interacting components that exchanges energy and matter with its surroundings. Inputs into the biosphere include energy (usually in the form of sunlight) and matter (such as carbon, nutrients, and water). Organisms use these inputs to function and grow. In the process, they create an amazing variety of outputs, some of which have important influences on geologic processes. At a local scale—such as that of a water-filled pore within loose sediment particles—a small group of organisms may have a geologic effect that is limited to a particular sedimentary environment. At larger scales, the activities of organisms may influence the concentrations of gases in the atmosphere or the cycling of certain elements through Earth’s crust.
Ecosystems
Think of a class project in which each member of a team has special skills that allow the team as a whole to exceed the capabilities of individuals working alone. Groups of organisms act in similar ways: individual organisms play roles that contribute to the survival of other organisms as well as their own. In the case of human groups, we accomplish this teamwork as a result of conscious decisions. For the organisms living together in a particular environment—referred to as a community—it happens through trial and error and involves feedbacks between the community and individuals. These feedbacks determine the structure and functioning of the community.
Whether at local, regional, or global scales, the interactions of biological communities with their environments define organizational units known as ecosystems. Ecosystems are composed of biological and physical components that function in a balanced, interrelated fashion. Ecosystems occur at many different scales (Figure 11.1). They may be separated by geologic barriers such as mountains, deserts, or oceans at the largest scale, or by barriers such as different water temperatures within a single hot spring at a much smaller scale (see the chapter opening photo). But no matter how large or small they are, all ecosystems are characterized by a flow, or flux, of energy and matter between organisms and their environment.
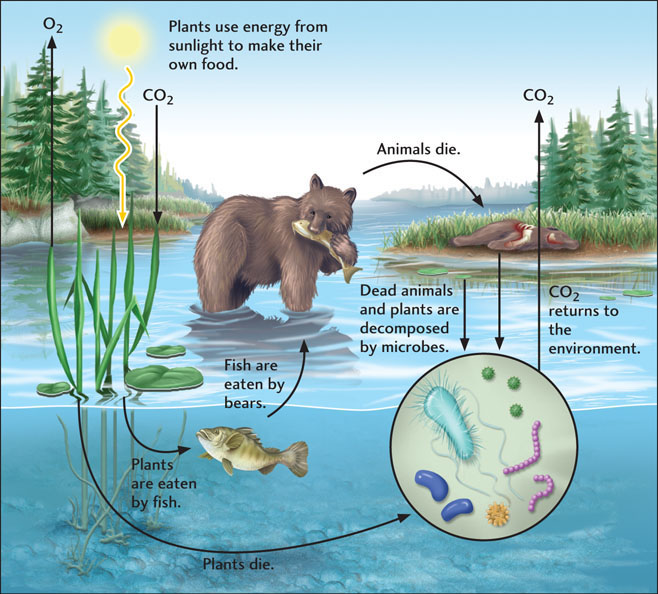
A typical ecosystem might involve, say, a river and its surroundings, where different groups of organisms are adapted to live in the water (fish), in the sediment (worms, snails), on the banks (grass, trees, muskrats), and in the sky above (birds, insects). In one sense, the river controls where the organisms live by supplying the ecosystem with water, sediment, and dissolved mineral nutrients. Conversely, the organisms influence how the river behaves; for example, grass and trees stabilize the riverbanks against the destructive effects of floods. The balance between such biologically controlled and geologically controlled processes ensures the long-term stability of the ecosystem.
285
Ecosystems respond sensitively to biological changes, such as the introduction of new groups of organisms. When severe imbalances in ecosystems occur, responses are often dramatic. Consider the effects of introducing a new organism into your neighborhood environment, such as a pretty new plant for your garden. In all too many cases, the new organism is better suited for its new environment than the current inhabitants and becomes invasive, multiplying rapidly and squeezing out the previous inhabitants (Figure 11.2). A successful invader often comes from a place where the physical environment is similar but where biological competition is less intense, so it is likely to win the battle for nutrients and space in its new home. Organisms that are squeezed out may become extinct if they are outcompeted by the invader in all the regions they formerly occupied.

Earth’s history shows us that ecosystems respond sensitively to geologic processes as well. Impacts by meteorites, huge volcanic eruptions, and rapid global warming are just a few of the processes that have contributed to the extinction of major groups of organisms. We will explore some of their effects later in this chapter.
Inputs: The Stuff Life Is Made Of
The organisms of any ecosystem can be subdivided into producers and consumers according to the way they obtain their food, which is their source of energy and nutrients (Table 11.1). Producers, or autotrophs, are organisms that make their own food. They use energy from sunlight or, in some cases, energy derived from chemicals in their environment to manufacture organic compounds such as carbohydrates. Consumers, or heterotrophs, get their food by feeding directly or indirectly on producers.
| Type | Energy Source | Carbon Source | Example |
|---|---|---|---|
| Photoautotroph | Sun | CO2 | Cyanobacteria |
| Photoheterotroph | Sun | Organic compounds | Purple bacteria |
| Chemoautotroph | Chemicals | CO2 | H, S, Fe bacteria |
| Chemoheterotroph | Chemicals | Organic compounds | Most bacteria, fungi, and animals, including humans |
It is often said that you are what you eat, and that statement is true not only for humans, but for all organisms. Our foods are all made up of more or less the same materials: molecules composed of carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur. So it doesn’t matter whether the organism is an autotroph or a heterotroph, it still utilizes the same six elements as food. What differs is the form (that is, the molecular structure) their food comes in. When heterotrophs such as humans eat bread, we are feeding ourselves on carbohydrates: large molecules formed of carbon, hydrogen, and oxygen. Even the lowliest microorganisms dine on carbon-bearing molecules such as carbon dioxide (CO2) or methane (CH4). The difference is that what is food for a microorganism may not seem like food to us!
286
Carbon
The fundamental building block of all life on Earth is carbon. On our planet, wherever life is present, carbon must be present. If water is removed, the composition of all organisms, including humans, is dominated by carbon. No other chemical element can match carbon in the variety and complexity of the compounds it can form. Part of the reason for its versatility is that it can form four covalent bonds with itself and other elements (see Figure 3.3), which allows for a wide variety of structures. Carbon acts as the template around which all organic molecules, such as carbohydrates and proteins, are built. Thus, carbon is critically important to organisms because it goes into manufacturing every part of them, from genes to body structures.
The biosphere largely controls the flux of carbon through the Earth system. Marine organisms extract carbon—which is present in seawater as dissolved CO2—to form carbonate shells and skeletons. When the organisms die, their skeletons settle to the seafloor, where they accumulate as sediments, effectively moving carbon from the biosphere to the lithosphere. The accumulation of the organic remains of organisms in freshwater wetlands and on the seafloor also moves carbon from the biosphere to the lithosphere. Over geologic time, these organic remains are transformed into oil, natural gas, and coal deposits. Today, when we extract and burn these deposits, we are moving carbon from the lithosphere to the atmosphere in the form of CO2 emissions.
Nutrients
Nutrients are chemical elements or compounds that organisms require to live and grow. Common plant nutrients include the elements phosphorus, nitrogen, and potassium—the ones most commonly found in garden fertilizer. Other organisms also depend on iron and calcium. Some organisms can manufacture their own nutrients, but others must obtain them in their diets from materials in their environment. Some specialized microorganisms can obtain nutrients by dissolving minerals.
Water
Life as we know it requires water (H2O). All organisms on Earth, including humans, are composed primarily of water, typically 50 to 95 percent. It is well known that humans can live for weeks without food, but most will perish in a few days without water. Even microorganisms that live in the atmosphere must obtain water from tiny droplets that condense around dust particles, and viruses must obtain water from their hosts.
Water is the habitat in which life first emerged and in which much of it still thrives. Water’s chemical properties and the way it responds to changes in temperature make it an ideal medium for biological activity. The cells of all organisms are made up primarily of an aqueous solution that promotes the chemical reactions of life. Water also helps moderate Earth’s climate, which has supported life for at least 3.5 billion years (see Chapter 15). Water is such an important ingredient for life that the search for extraterrestrial life must begin with the search for water, as we will see at the end of this chapter.
Energy
All organisms need energy to live and grow. Some of the simplest organisms, such as single-celled algae, obtain energy from sunlight. Others acquire energy by breaking down chemicals in their environment. Heterotrophs get energy by feeding on other organisms. Energy is important because it fuels the conversion of simple molecules such as carbon dioxide and water into larger molecules, such as carbohydrates and proteins, which are essential for life.
Processes and Outputs: How Organisms Live and Grow
Metabolism encompasses all the processes organisms use to convert inputs into outputs. In one type of metabolic process, organisms use small molecules, such as CO2, H2O, and CH4, and energy to create larger molecules, such as proteins and certain types of carbohydrates, that enable them to function and grow. Other carbohydrates—for example, a sugar called glucose—are stored for later use as an energy source—that is, food. In another type of metabolic process, organisms break down food to release the energy it contains.
287
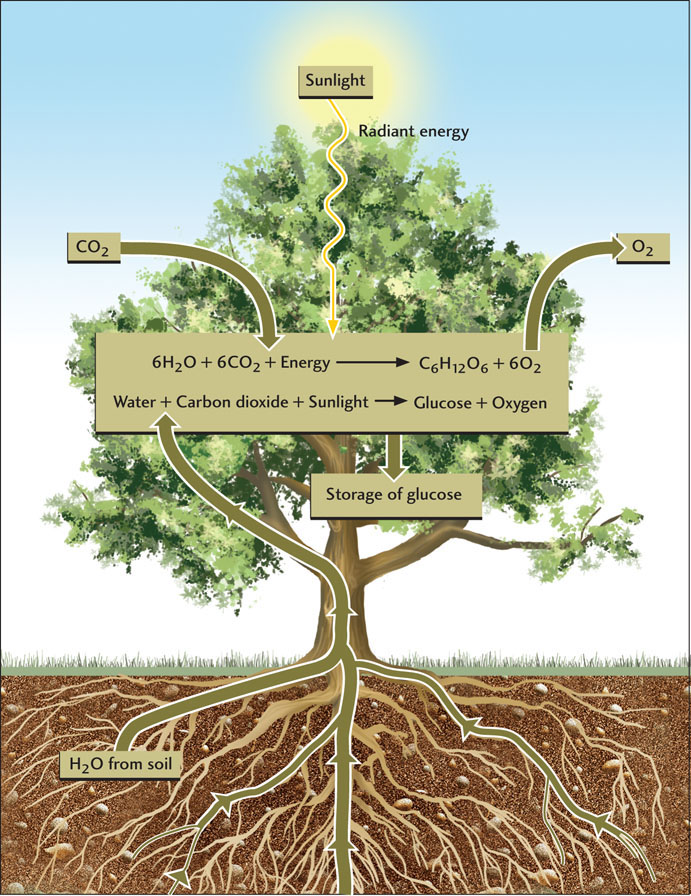
One particularly well-known metabolic process is photosynthesis (Figure 11.3). Through this process, organisms such as plants and algae use energy from sunlight to convert water and carbon dioxide into carbohydrates (such as glucose) and oxygen (Table 11.2). This reaction proceeds as follows:
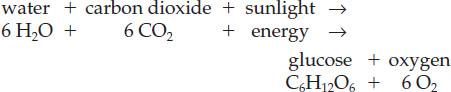
| Photosynthesis | Respiration |
|---|---|
| Stores energy as carbohydrates | Releases energy from carbohydrates |
| Uses CO2 and H2O | Releases CO2 and H2O |
| Increases mass | Decreases mass |
| Produces oxygen | Consumes oxygen |
288
The oxygen is released into the atmosphere, and the glucose is stored as an energy source for future use by the organism. An important group of microorganisms known as the cyanobacteria also use photosynthesis to make carbohydrates; in fact, they probably originated the process early in life’s history.
The other key metabolic process is respiration, by which organisms release the energy stored in carbohydrates such as glucose (see Table 11.2). All organisms use oxygen to burn, or respire, carbohydrates to release energy, but different organisms respire in different ways. For example, humans and many other organisms consume oxygen gas (O2) from the atmosphere to metabolize carbohydrates, and they release carbon dioxide and water as by-products. In this case, the reaction is the reverse of photosynthesis:
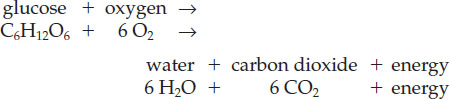
But other organisms, such as microorganisms that live in environments where oxygen is absent, have a more difficult task. They must break down oxygen-containing compounds dissolved in water, such as sulfate (SO4−2), to obtain oxygen. During the course of these reactions, various gases—such as hydrogen (H2), hydrogen sulfide (H2S), and methane (CH4)—may be produced as by-products.
The metabolism of organisms affects the geologic components of their environment. For example, the oxygen released by photosynthesis reacts with iron-bearing silicate minerals such as pyroxene and amphibole to form iron-bearing oxide minerals such as hematite (see Chapter 16). When organisms produce CO2 and CH4, they escape to the atmosphere and contribute to global warming. Conversely, when organisms consume these gases, they contribute to global cooling.
Biogeochemical Cycles
In the course of living and dying, organisms continuously exchange energy and matter with their environment. This exchange occurs at the scale of the individual organism, the ecosystem of which it is a part, and the global biosphere. The metabolic consumption and production of gases such as CO2 and CH4 is a good example of how organisms may exert global controls on Earth’s climate. Carbon dioxide and methane are greenhouse gases: gases that absorb heat emitted by Earth and trap it in the atmosphere. When organisms produce more CO2 and CH4 than they consume, the climate will warm; when they consume more CO2 and CH4 than they produce, the climate will cool. The concentration of greenhouse gases in the atmosphere is not the only control on global climates, as we will learn in Chapter 15, but it is an important one that directly involves the biosphere.
Geobiologists keep track of exchanges between the biosphere and other parts of the Earth system by studying biogeochemical cycles. A biogeochemical cycle is a pathway by which a chemical element or compound moves between the biological (“bio”) and environmental (“geo”) components of an ecosystem. The biosphere participates in biogeochemical cycles through the inflow and outflow of atmospheric gases by respiration, the inflow of nutrients from the lithosphere and hydrosphere, and the outflow of those nutrients through the death and decay of organisms.
Because ecosystems vary in scale, so do biogeochemical cycles. Phosphorus, for example, may cycle back and forth between the water and the microorganisms in the pores of sediments, or it may cycle back and forth between uplifted rocks in mountains and the sediments deposited along the margins of ocean basins (Figure 11.4). In either case, when phosphorus-containing organisms die, the phosphorus may accumulate in a temporary repository before being recycled. Sediments and sedimentary rocks are an important repository for this element.
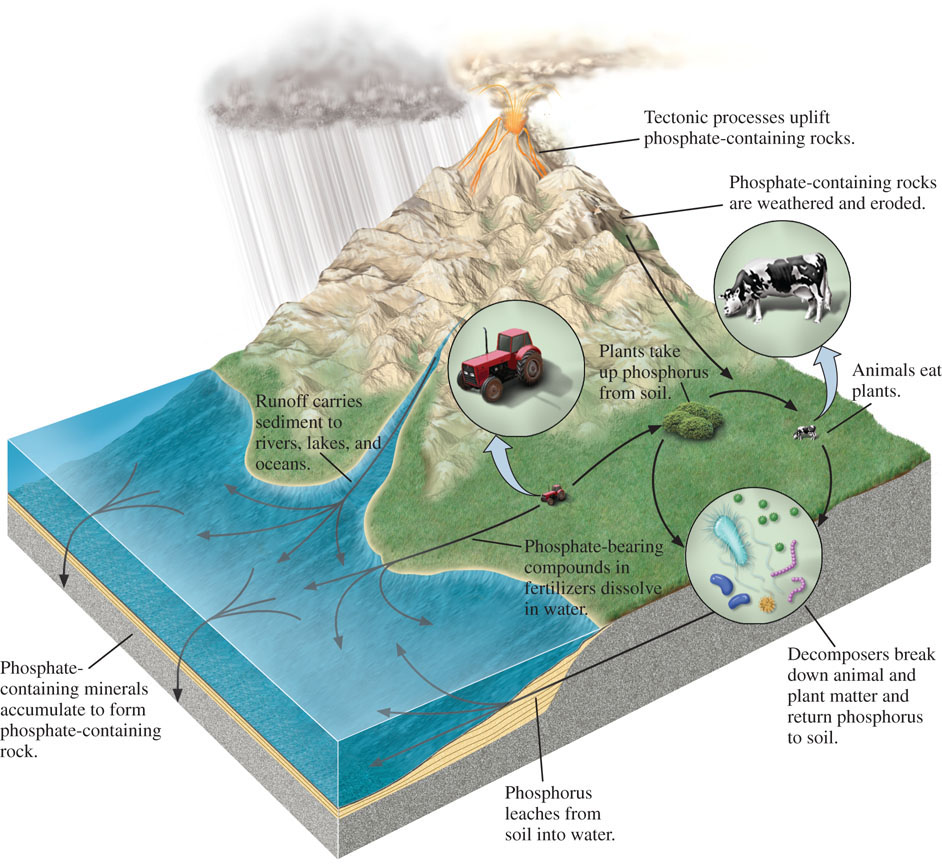
Knowledge of biogeochemical cycles is important for understanding the mechanisms associated with major geobiological events throughout Earth’s history, as we will see later in this chapter. It is also critical for understanding how elements and compounds that humans emit into the atmosphere and ocean are interacting with the biosphere, as we will see in Chapters 15 and 23.